The advent of high-performance solid-state batteries has stirred significant interest in the fields of energy storage and materials science. Central to the development of these innovative batteries are lithium and sodium metal anodes, which hold the promise of transforming energy storage capabilities. However, to unlock their full potential, a thorough understanding of their microstructure is imperative. Recent advancements by an international research collaboration, including Justus Liebig University Giessen (JLU) in Germany, the University of California, Santa Barbara, and the University of Waterloo in Canada, have made it possible to analyze the microstructural intricacies of electrochemically deposited lithium and sodium for the first time. This groundbreaking work, recently published in Nature Materials, heralds new strategies for optimizing the electrochemical performance of these alkali metals in batteries.
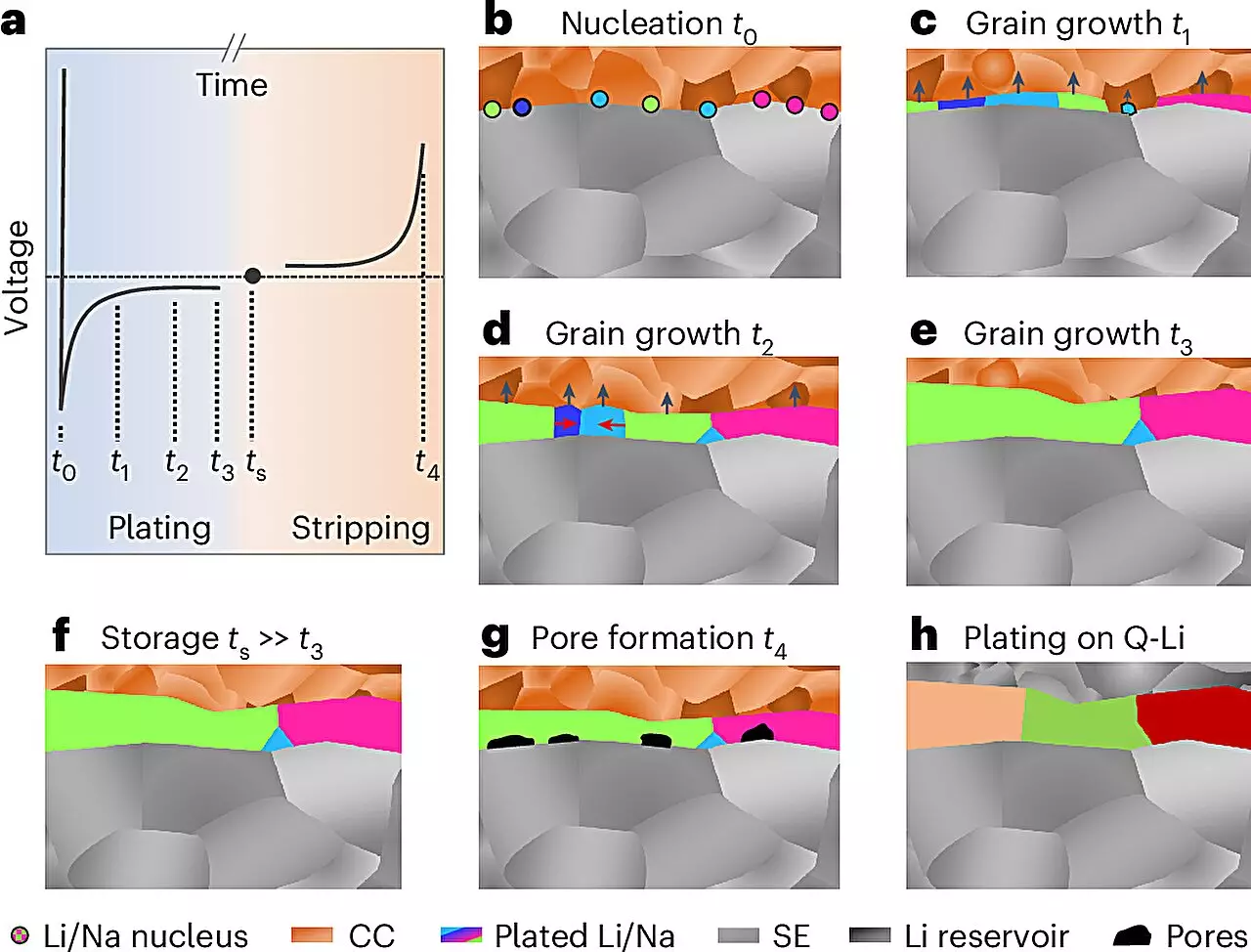
Microstructure refers to the arrangement and characteristics of materials on a scale ranging from nanometers to micrometers. This internal architecture significantly influences a material’s electrochemical behavior, which is critical for applications in energy storage. For most metals utilized in technology today, the microstructure has been well-studied, allowing for tailored modifications to enhance desired properties. In stark contrast, lithium and sodium present unique challenges due to their highly reactive nature. Their surfaces form robust reaction layers almost instantaneously when exposed to air or other environments, creating barriers for assessing their microstructures. Therefore, the inability to probe the internal structure of alkali metals has impeded progress in the design of efficient battery systems.
The collaborative team led by Prof. Dr. Jürgen Janek from JLU developed a meticulous methodological approach involving low-temperature preparation and analysis within inert gas environments. This innovative technique culminated in the application of electron backscatter diffraction (EBSD), a powerful imaging tool that enabled the researchers to visualize the microstructure of lithium and sodium layers up to 100 micrometers thick. Janek expressed astonishment at the grain size observed in the formed metal layers, stating that the findings significantly contribute to understanding the growth mechanisms of these metals. Furthermore, the insights gained regarding sodium metal are expected to catalyze further research within POLiS (Post Lithium Energy Storage), focusing on the advancement of sodium batteries.
Challenges and Future Prospects in Solid-State Battery Development
Solid-state batteries are touted for their capacity to provide higher energy density, enhanced safety profiles, and extended operational lifetimes. The integration of alkali metal electrodes, particularly in combination with ceramic solid electrolytes, is a critical component of this technology’s evolution. Nonetheless, considerable challenges remain. One major hurdle is the susceptibility of lithium and sodium metals to deformation during electrochemical cycling, a phenomenon that adversely impacts both charging and discharging processes. This deformation can lead to the formation of pores within the metal during discharge cycles and the growth of dendritic structures during charging, potentially leading to hazardous short-circuiting events.
To ensure the practicality of lithium or sodium metal electrodes, it is essential that these metals form solely during the initial charging phase, thus negating complications associated with handling reactive alkali metal foils. The global research community has invested substantially in identifying solutions to these challenges over the past decade. JLU’s research team, led by Prof. Janek, is among the preeminent groups conducting such inquiries, collaborating harmoniously with counterparts in California and Canada to breakthrough long-standing barriers.
The pioneering work in elucidating the microstructure of lithium and sodium anodes represents a pivotal step forward for solid-state battery technology. By overcoming the historical complications associated with analyzing these reactive metals, the research team has opened up new avenues for enhancing battery performance. The ability to finely manipulate the microstructure of alkali metals could unlock unprecedented efficiency and safety levels in energy storage systems, ultimately contributing to a sustainable energy future. As technological demands for smarter and more efficient batteries continue to grow, this research lays a crucial foundation for the next generation of solid-state batteries, pushing the boundaries of what is feasible in the electrochemical landscape.

Leave a Reply