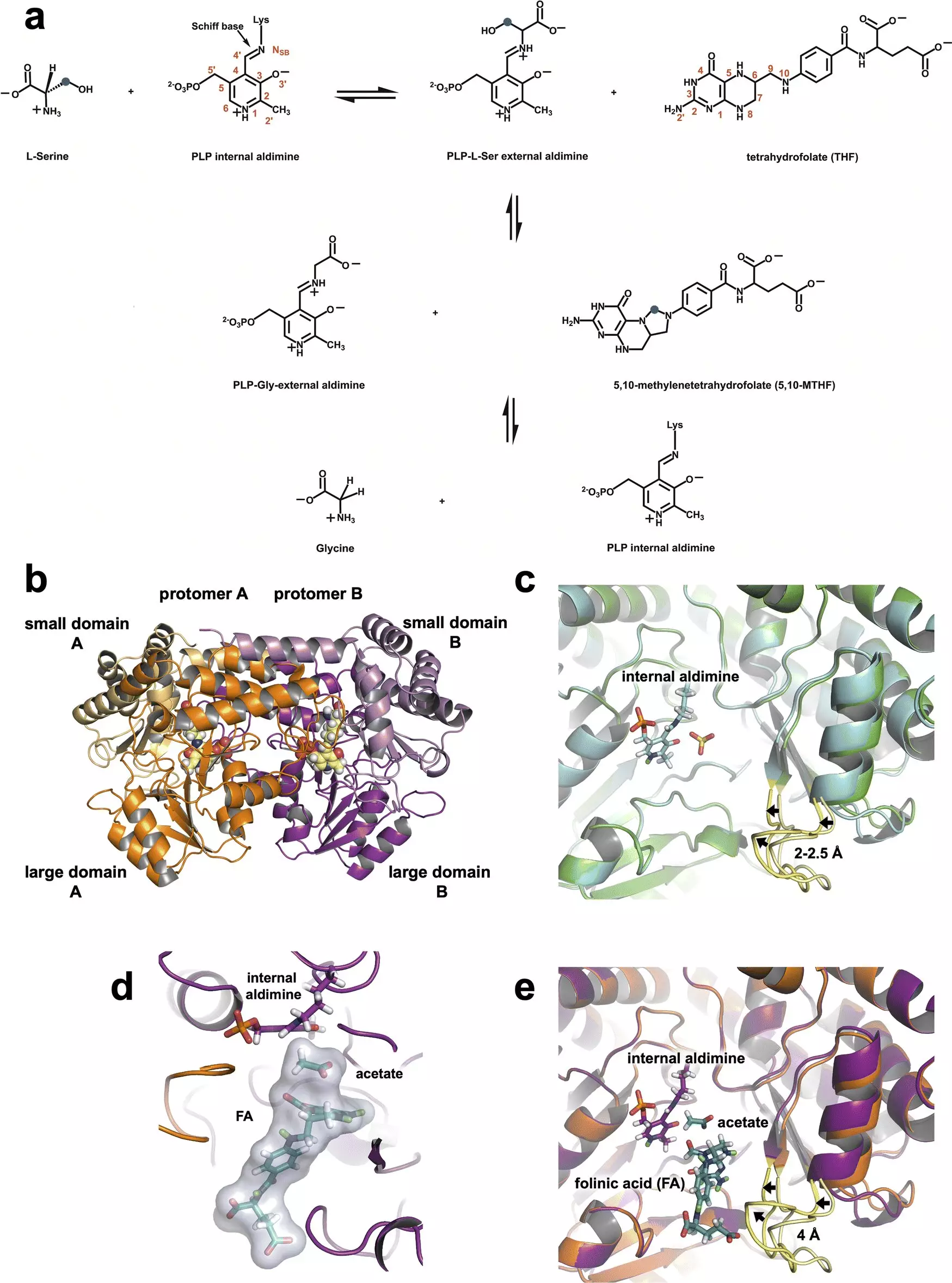
Recent advancements in neutron imaging at the Oak Ridge National Laboratory (ORNL) have provided a vital understanding of serine hydroxymethyltransferase (SHMT), an enzyme crucial to cellular metabolism. This research could be pivotal in developing targeted therapies for aggressive cancers. Unlike traditional methods, which often rely on slower techniques, this study utilized neutrons in two distinct experiments to elucidate the enzyme’s atomic structure and its catalytic function. By capturing precise atomic details, scientists have laid the groundwork for innovative drug design that could potentially disrupt cancer’s unchecked growth.
SHMT plays a key role in one-carbon metabolism, a biochemical pathway that significantly contributes to cell division. In normal conditions, SHMT converts serine into glycine, moving a carbon atom to tetrahydrofolate, a critical component of DNA and RNA synthesis. However, in cancer cells, SHMT and other enzymes are often overexpressed, creating an environment where cancer cells multiply uncontrollably. The ability to inhibit SHMT may not only slow down this process but may also serve as a therapeutic approach to halt cancer’s progression at an early metabolic stage. By manipulating the flow of this metabolic pathway, drug design could effectively starve cancer cells.
The innovative approach of combining neutron diffraction with conventional X-ray techniques has propelled this research forward. Neutrons are particularly adept at detecting light elements, such as hydrogen, integral to understanding protein structure and function. Co-author Robert Phillips emphasized the significance of retaining the hydrogen atom in glutamate during chemical reactions. This dual role of glutamate as both an acid and base is crucial for SHMT’s catalytic activity, moving protons between substrates and activating the chemical process necessary for metabolism.
In this study, the researchers capitalized on the strengths of both neutron and X-ray methods to analyze SHMT’s protein structure at physiologically relevant temperatures. By focusing laser precision on the proton’s position, the team was able to correlate the presence of hydrogen with the enzyme’s catalytic efficacy, thereby painting a more vivid picture of its operational dynamics.
Understanding the placement and roles of hydrogen atoms within enzymes is pivotal for drug design. The knowledge of how protonation states influence chemical behavior enables researchers to develop molecules that can effectively inhibit enzyme activity. As stated by Andrey Kovalevsky, the implications of this research extend into the realm of small-molecule inhibitors, which can target SHMT directly. By mimicking substrates, such inhibitors could knock SHMT out of action, thereby impeding the cascade of metabolic reactions that support cancer cell proliferation.
Moreover, these findings have broader implications for developing treatments aimed at earlier intervention in cancer pathways. Existing cancer drugs already target other enzymes in the one-carbon metabolic pathway; however, SHMT offers a unique advantage due to its positioning in this pathway, signifying an opportunity to halt malignancies before they gain momentum.
The Challenges of Targeting Cancer
One of the most formidable challenges in cancer treatment is the highly adaptive nature of cancer cells. Unlike antibiotic resistance seen in bacterial infections, where pathogens counteract treatment strategies by altering their metabolism or structure, cancer cells recalibrate their metabolic processes to ensure continuity of growth even when specific pathways are interfered with. Consequently, developing drugs that specifically target cancer cells while sparing normal cells poses a significant challenge.
The groundwork laid by the study of SHMT allows researchers to tailor inhibitors that specifically affect cancerous activity. Since existing therapies often target both cancerous and healthy cells, leading to severe side effects, the insights garnered from this research could lead to more precise treatments that minimize patient discomfort.
The Future of Enzyme Research and Drug Design
The advancements made with neutron technology signal a promising horizon for cancer treatment. According to William Nelson from Johns Hopkins University, combining AI with genetic sequencing may soon allow scientists to expedite drug discovery processes. While this ideal scenario is still in the realm of aspiration, the work done with SHMT sets a strong foundation for future research.
Ultimately, the neutron studies at ORNL not only advance our understanding of SHMT but may also revolutionize how we approach cancer therapies. By dissecting the intricate relationships within metabolic pathways, researchers can target specific mechanisms to impede cancer’s growth, potentially crafting solutions that are as innovative as they are effective. As the field evolves, the intersection of queue-busting techniques like AI and insights into enzyme functionality will undoubtedly refine cancer treatment strategies, making them more efficient and less harmful to patients.

Leave a Reply