Exploring the inner workings of cellular structures has always been a challenging task for researchers. However, with the advancement of technology, specifically cryo-electron tomography (cryo-ET), scientists are now able to visualize and analyze cellular components in their natural environment with remarkable precision. A recent study conducted by researchers at the MPI of Biochemistry in Martinsried and the University Medical Center Göttingen sheds light on the intricate process of protein folding with the help of cryo-ET.
Proteins are essential molecules that carry out various functions within the cell, and in order to perform these functions effectively, they must adopt specific three-dimensional structures. Protein folding helpers, known as chaperonins, play a critical role in ensuring that newly synthesized proteins achieve their correct functional form. These chaperonins assist in the folding process, preventing misfolding which can lead to diseases such as Alzheimer’s and Parkinson’s. Understanding the structure and function of chaperonins is vital for developing strategies to combat these diseases.
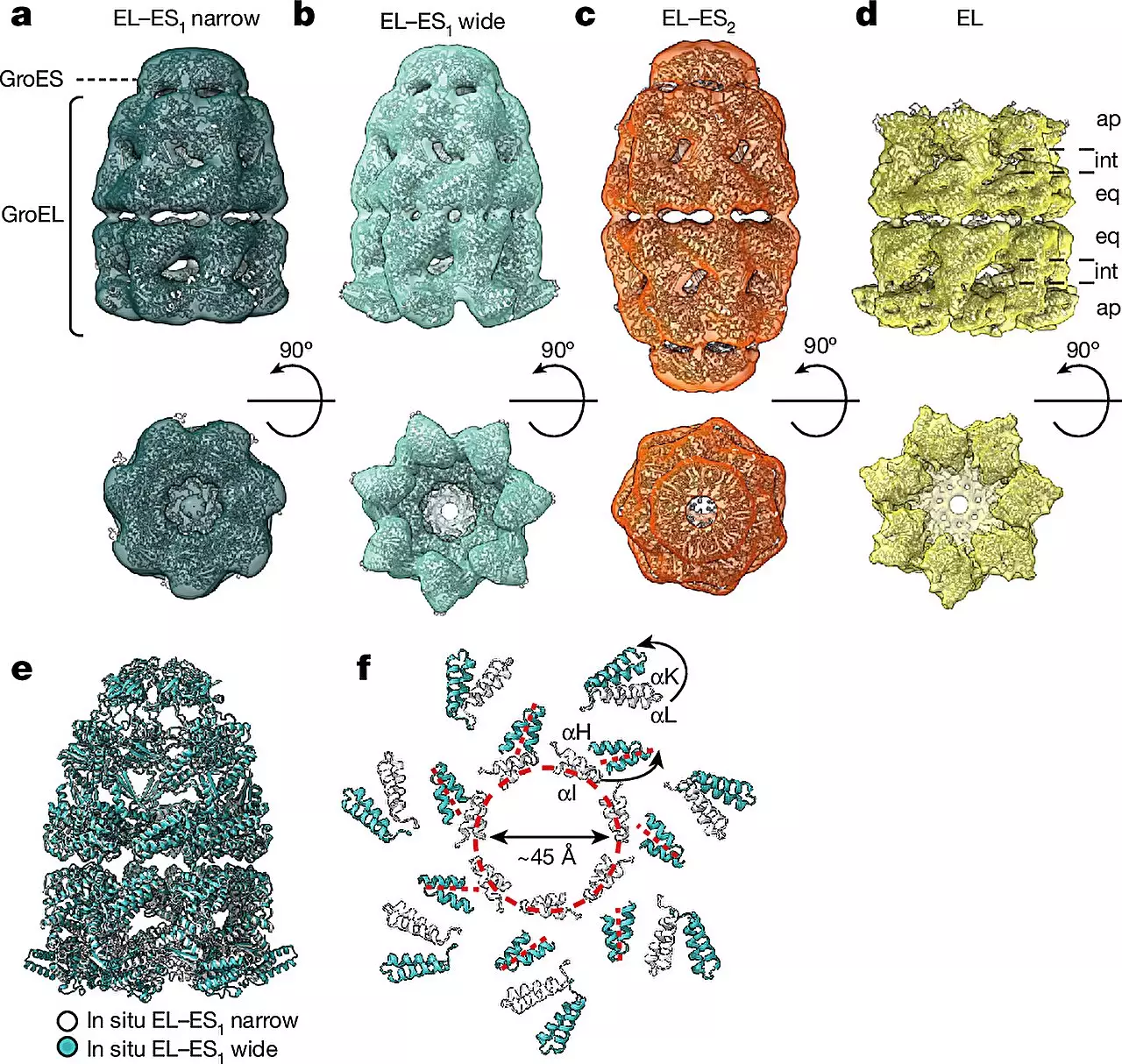
By utilizing cryo-ET, researchers were able to delve into the world of chaperonin complexes in the bacterium E. coli with unparalleled detail. The study revealed the dynamic nature of chaperonin activity, showcasing conformational changes within the complex as well as its interactions with client proteins inside the folding chambers. Two main forms of the GroEL-GroES complex, referred to as “bullet” and “football,” were identified within the cells, each displaying distinct structural symmetries. This observation provides valuable insights into the mechanisms underlying protein folding in living organisms.
To enhance their analysis, the researchers combined cryo-ET with single-particle cryo-electron microscopy (cryo-EM) and quantitative mass spectrometry. This multi-faceted approach enabled them to observe different conformations of chaperonin complexes in diverse cellular states and quantify their abundance. By visualizing these complexes directly within the bacterium, as opposed to in a controlled laboratory setting, the researchers achieved a significant breakthrough in the field of structural biology. This advancement was made possible by the minute size of the chaperonin complex, measuring only 14 nanometers wide.
Decades of experiments with purified chaperonin complexes have yielded conflicting results on their mode of action. The discrepancies observed can be attributed to the limitations of in vitro experiments, which fail to replicate the complex conditions present inside living cells. Through cellular cryo-ET, researchers can now bridge this gap by visualizing chaperonin complexes in their native cellular environment at high resolutions. This novel approach has provided crucial insights into the dynamic assembly of chaperonins during the protein folding process.
The findings of this study suggest that chaperonin complexes undergo distinct structural transformations, transitioning between the asymmetric “bullet” and symmetric “football” shapes during a single reaction cycle. This discovery paves the way for further investigations into the intricate mechanisms governing protein folding and opens new avenues for therapeutic interventions in diseases associated with protein misfolding.
The integration of cryo-ET and advanced imaging techniques has revolutionized our understanding of protein folding processes within living cells. By unraveling the complexities of chaperonin activity, researchers are poised to make significant strides in the field of structural biology and potentially develop innovative strategies for combating protein misfolding diseases.

Leave a Reply