Water, a simple molecule with profound complexities, continues to intrigue scientists with its unique properties. One such property is the behavior of water molecules at an interface with air, where they lose their energy in a rather fascinating manner. Researchers at RIKEN have delved deep into this phenomenon to gain a better understanding of the processes that take place at water surfaces.
The Role of Hydrogen Bonds
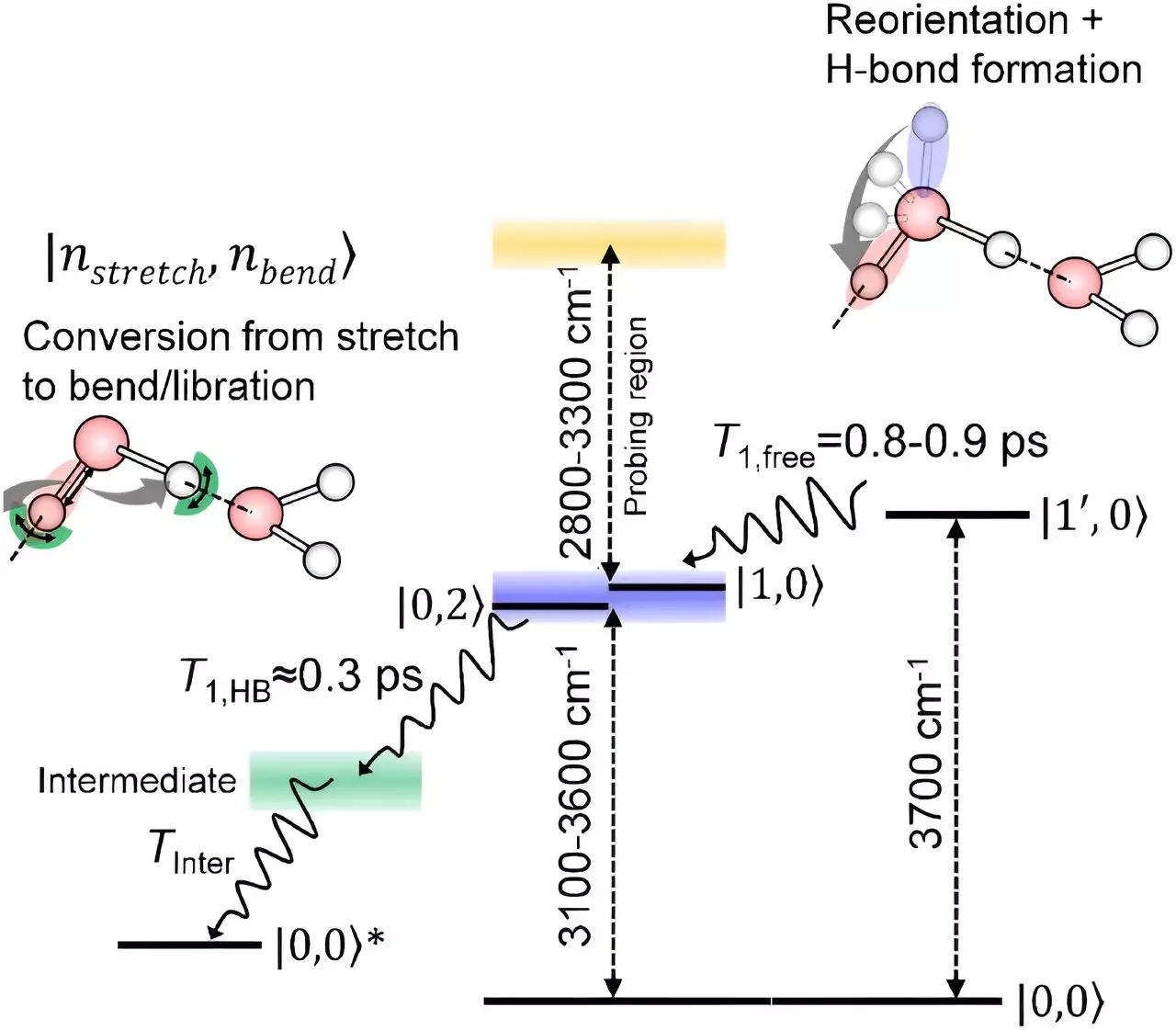
At the heart of water’s peculiar behavior are hydrogen bonds – weak attractions that form between neighboring water molecules due to the electronegativity of oxygen. This results in a slightly negative oxygen atom being attracted to the slightly positive hydrogen atoms in adjacent molecules. However, for water molecules at the surface, this bond formation is different.
One of the biggest challenges in studying water molecules at the interface has been isolating the signals from these surface molecules. However, the team at RIKEN led by Tahei Tahara has made significant strides in this area by developing advanced spectroscopy techniques. Their latest technique, based on infrared spectroscopy, has provided insights into how the oxygen-hydrogen bonds of surface water molecules relax.
Through their innovative approach, the researchers found that the oxygen-hydrogen bonds sticking up into the air rotate initially without losing energy. Subsequently, they relax in a manner similar to the molecules in the bulk liquid, which form a network of hydrogen bonds. This revelation challenges the conventional belief that surface water molecules behave distinctly from those inside the liquid.
A Comprehensive Understanding
The findings from this study offer a comprehensive picture of how the stretching of oxygen-hydrogen bonds relaxes at the surface of water. Tahara emphasizes that the relaxation process is surprisingly similar for molecules at the interface and those within the liquid after interacting with their neighbors. This insight sheds light on a previously murky area of water surface interactions.
Building upon their spectroscopic success, Tahara and his team plan to investigate chemical reactions that occur at the interface of water. By applying their technique to these reactions, they hope to unravel more mysteries surrounding the behavior of water molecules at interfaces with other substances.
The study conducted by RIKEN scientists has provided invaluable insights into the behavior of water molecules at the interface with air. By overcoming technical challenges and developing sophisticated spectroscopy techniques, the researchers have unveiled a new understanding of how these molecules lose their energy. This knowledge not only enhances our comprehension of water’s unique properties but also paves the way for further research in related fields.

Leave a Reply