Recent advances in the field of sustainable chemistry have garnered significant attention, particularly as societies grapple with the need for environmentally friendly solutions amid escalating climate change challenges. A groundbreaking study published in *Nature Energy* explores the dynamic processes involved in converting carbon dioxide (CO2) into commercially valuable chemicals such as ethylene and ethanol. Led by Dr. Arno Bergmann, along with renowned colleagues Prof. Dr. Beatriz Roldán Cuenya and Prof. Dr. Núria López, this research employs cutting-edge spectroscopic techniques to elucidate the complex mechanisms underpinning the electrochemical reduction of CO2 (CO2RR). The implications of these discoveries could be revolutionary for the chemicals industry.
The Importance of CO2 Reduction
CO2 emissions have been identified as a principal driver of global warming, leading to an urgent need for innovative strategies that can both mitigate these emissions and produce useful materials. The process of CO2RR offers a promising avenue by harnessing renewable electricity to transform CO2 into high-value chemicals, effectively creating a circular carbon economy. Ethylene, a precursor for environmentally friendly plastics, and ethanol, a renewable fuel source, are two focal products of this process. Despite their significance, the precise pathways and intermediate compounds involved in their production have remained ambiguous—until now.
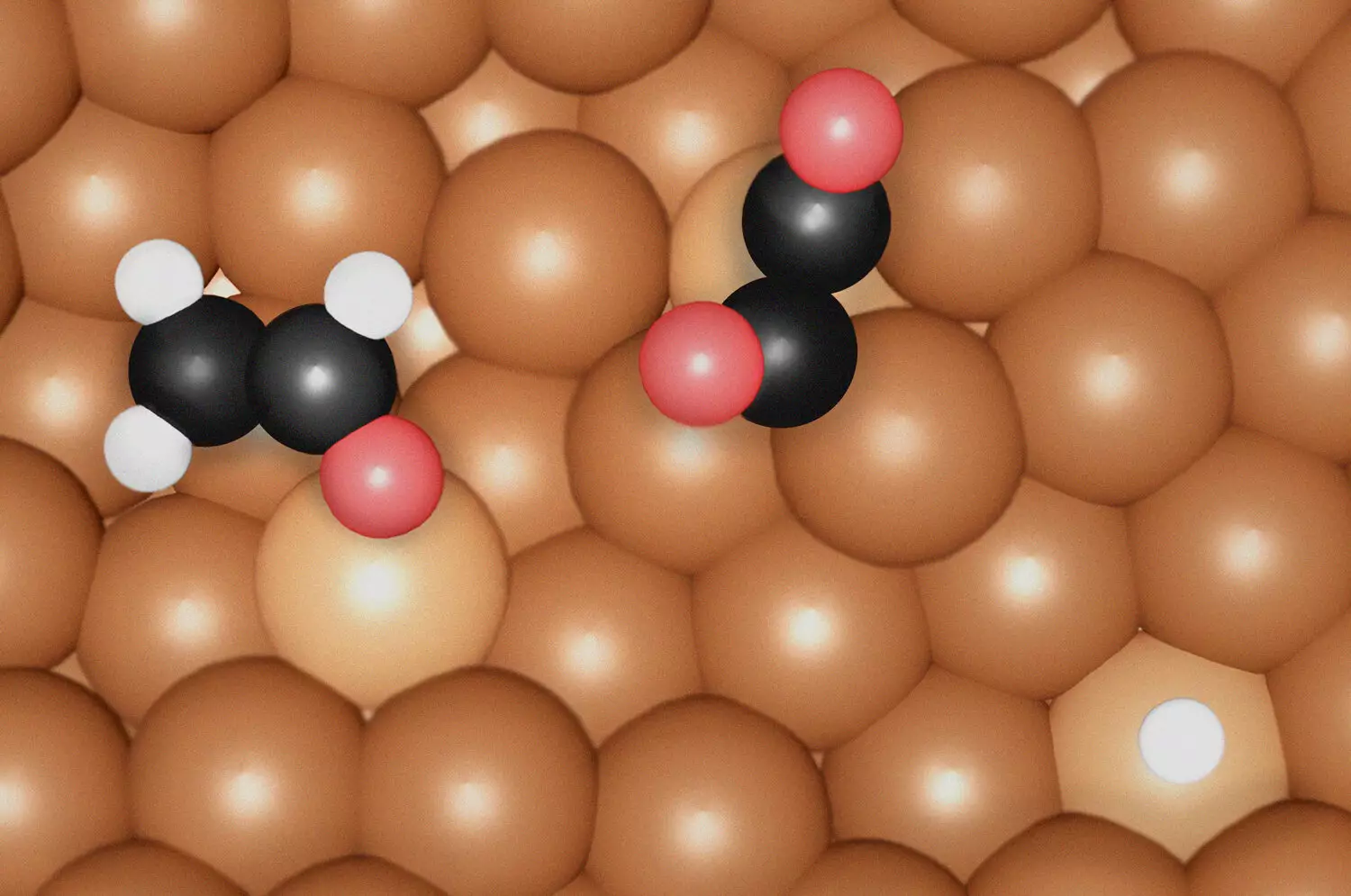
The study employed an ingenious combination of in-situ surface-enhanced Raman spectroscopy (SERS) and density functional theory (DFT) to reveal molecular species on copper (Cu) electrocatalysts. This innovative approach allowed the research team to decipher the key intermediates and active sites facilitating CO2 reduction. Their findings indicate a clear distinction in the mechanisms for the formation of ethylene and ethanol. Ethylene synthesis primarily relies on the formation of *OC-CO(H) dimers at undercoordinated Cu sites, whereas ethanol production necessitates a more complex coordination environment characterized by *OCHCH2 intermediates.
Significance of Surface Morphology
An unexpected but enlightening revelation of the research was the critical role played by the surface morphology of the Cu electrocatalysts. The team discovered that the undercoordinated Cu sites enhance the binding strength of carbon monoxide (CO), a pivotal step in the reduction process. These atomic-level irregularities, which potentially emerge during reaction conditions, facilitate a more effective catalytic surface. The correlation between surface structure and catalytic efficiency emphasizes the necessity for specific morphological attributes in the design of catalysts to optimize performance.
A Collaborative Endeavor in Science
The study’s success can be attributed to the collaborative effort of various research groups, notably one based in Spain. This partnership exemplifies the value of combining theoretical insights with experimental data to deepen the understanding of the CO2 reduction process. The comprehensive approach employed by the Interface Science Department at the Fritz Haber Institute and the Institute of Chemical Research of Catalonia marks a significant stride in this field. It fosters a deeper understanding of how specific conditions and intermediates impact CO2 conversion efficiency.
The insights gained from this study not only advance academic understanding but also hold profound practical implications. By pinpointing the intermediates and active sites essential for the efficient production of ethylene and ethanol, chemists can develop more sophisticated catalysts. This innovation could significantly improve the carbon footprint associated with chemical manufacturing processes, fostering a more sustainable future for the industry.
The intricate study of CO2 reduction unveiled critical aspects of how one can effectively convert greenhouse gases into valuable chemicals, paving the way for enhanced environmental sustainability. As researchers continue to unravel the complexities of these transformation processes, we edge closer to realizing the potential of CO2 as a renewable resource. The implications extend beyond academic circles, reaching industries dedicated to creating environmentally friendly technologies and processes, thus promising a future where sustainability and innovation go hand in hand.

Leave a Reply