High entropy oxides (HEOs) represent a fascinating class of materials that has garnered increasing attention in recent years due to their potential applications, particularly in electronic devices. Characterized by their intricate compositions that often encompass multiple transition metal oxides, these materials have unique electrochemical properties that set them apart from traditional ceramics and oxides. A recent study published in the *Journal of the American Chemical Society* has unveiled groundbreaking insights into how various synthesis strategies can significantly affect both the atomic structure and functional attributes of HEOs.
Alannah Hallas, a materials scientist associated with the University of British Columbia’s (UBC) Blusson Quantum Matter Institute, led this important investigation. Hallas emphasized the wide-ranging chemical versatility inherent in high entropy oxide systems, stating, “there is kind of a limitless possibility in the ways we can construct them.” This versatility not only allows for diverse configurations but also raises questions about how different preparation methods can influence material properties.
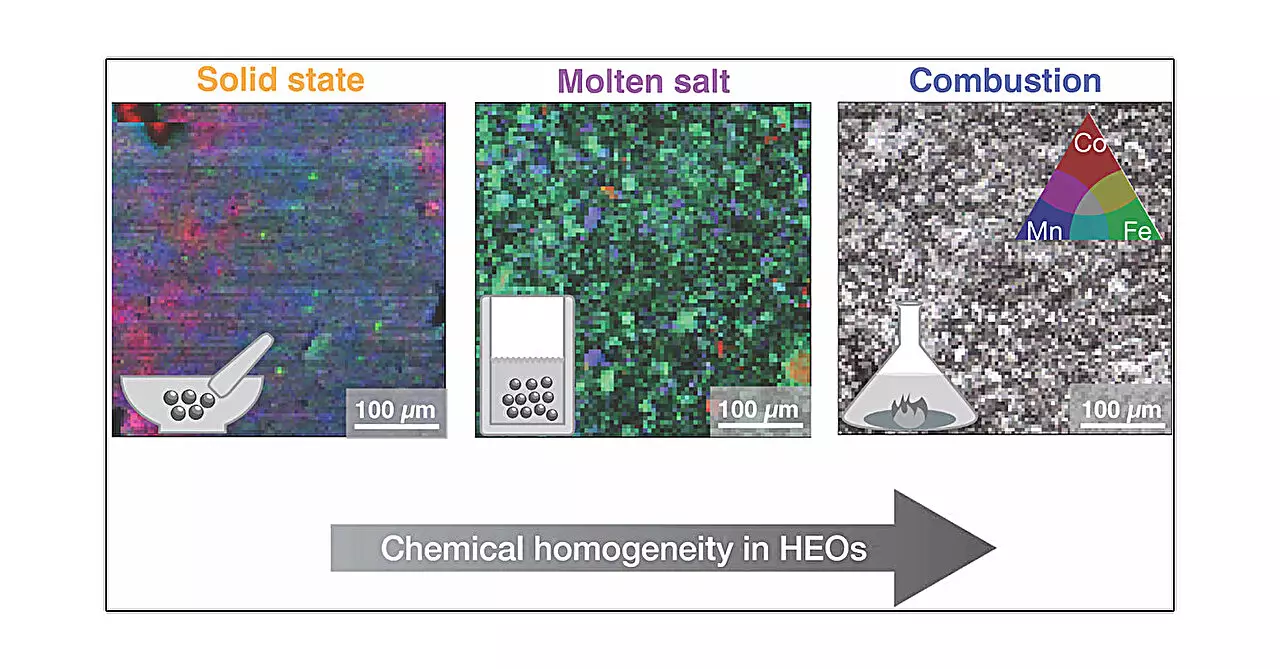
In the study, researchers prepared identical samples of a high entropy oxide with a spinel crystal structure using five distinct synthesis techniques: solid state, high pressure, hydrothermal, molten salt, and combustion synthesis. Each of these methods involves unique heating protocols, cooling rates, and chemical environments, contributing to the diverse microstructure and properties of the resulting materials.
For instance, the solid-state method involves mixing and heating metal oxides akin to baking a cake, where uniformity in composition often leads to predictable outcomes. Conversely, high-pressure synthesis employs elevated pressures, which can modify phase transitions and crystal formations. Hydrothermal synthesis mimics geological processes, utilizing high-temperature water to promote crystal growth under pressure, a method known for producing quality crystalline structures reminiscent of natural mineral formation.
Molten salt synthesis takes a different route by leveraging liquid metal salts that cool to precipitate crystalline materials as their temperature declines. Lastly, the combustion method ignites a gel formed from metal salts, leading to rapid transformations that can often stabilize unique microstructures. Each approach introduces specific conditions that yield varying local structures, a finding highlighted by lead author Mario Ulises González-Rivas.
The study revealed that even though the average structure of the samples remained relatively constant across different synthesis methods, there were notable variations in local structures and microstructures. Particularly, combustion synthesis yielded the most homogeneous samples, suggesting that the chosen synthesis technique plays a pivotal role in dictating the resultant material’s properties. This finding underscores the importance of precisely tuning synthesis methods for specific applications, especially in fields where material performance is critical, such as energy conversion and storage technologies.
González-Rivas pointed out, “Our results effectively provide a new optimization axis to be considered when implementing these materials in an applied setting.” This perspective is vital, as it allows scientists and engineers to tailor material properties to meet specific functional demands, thereby enhancing the performance of energy systems and electronic devices.
This pioneering research is the result of collaboration among various institutions, including the UBC Blusson QMI, the University of Saskatchewan, and the Max Planck Institute for Solid State Research. Such interdisciplinary partnerships are essential in advancing materials science, as they enable the pooling of expertise and resources necessary to tackle complex material challenges.
As researchers continue to explore the landscape of high entropy oxides, the implications of this study extend far beyond fundamental understanding. The capacity to manipulate material properties via synthesis opens new avenues for the development of high-performance materials that could revolutionize energy systems and electronic applications. Moving forward, the exploration of synthesis methodologies will likely lead to innovative approaches, driving the advancement of technology and addressing critical energy challenges of the future. Encouragingly, the scope of research in this area appears limitless, hinting at exciting developments that await on the horizon.

Leave a Reply