The field of biocatalysis has long been focused on optimizing natural enzyme functions for synthetic chemistry purposes. However, a recent study by UC Santa Barbara researchers, led by chemistry professor Yang Yang, has taken a groundbreaking approach by exploring entirely new enzymatic reactions through the use of photobiocatalysis. This innovative method leverages the power of light to drive chemical transformations, leading to the production of non-canonical amino acids that hold immense value as building blocks for various applications.
Photobiocatalysis represents a relatively young but promising field in chemistry, where enzymes are excited by light to facilitate energy transfer and generate free radicals that can catalyze complex reactions. By merging the selectivity and efficiency of enzymes with the versatility of light, researchers are able to devise novel processes that yield unique chemical products, such as non-canonical amino acids. This approach opens up a realm of possibilities for developing new therapeutics, bioactive compounds, and functional proteins with tailored properties.
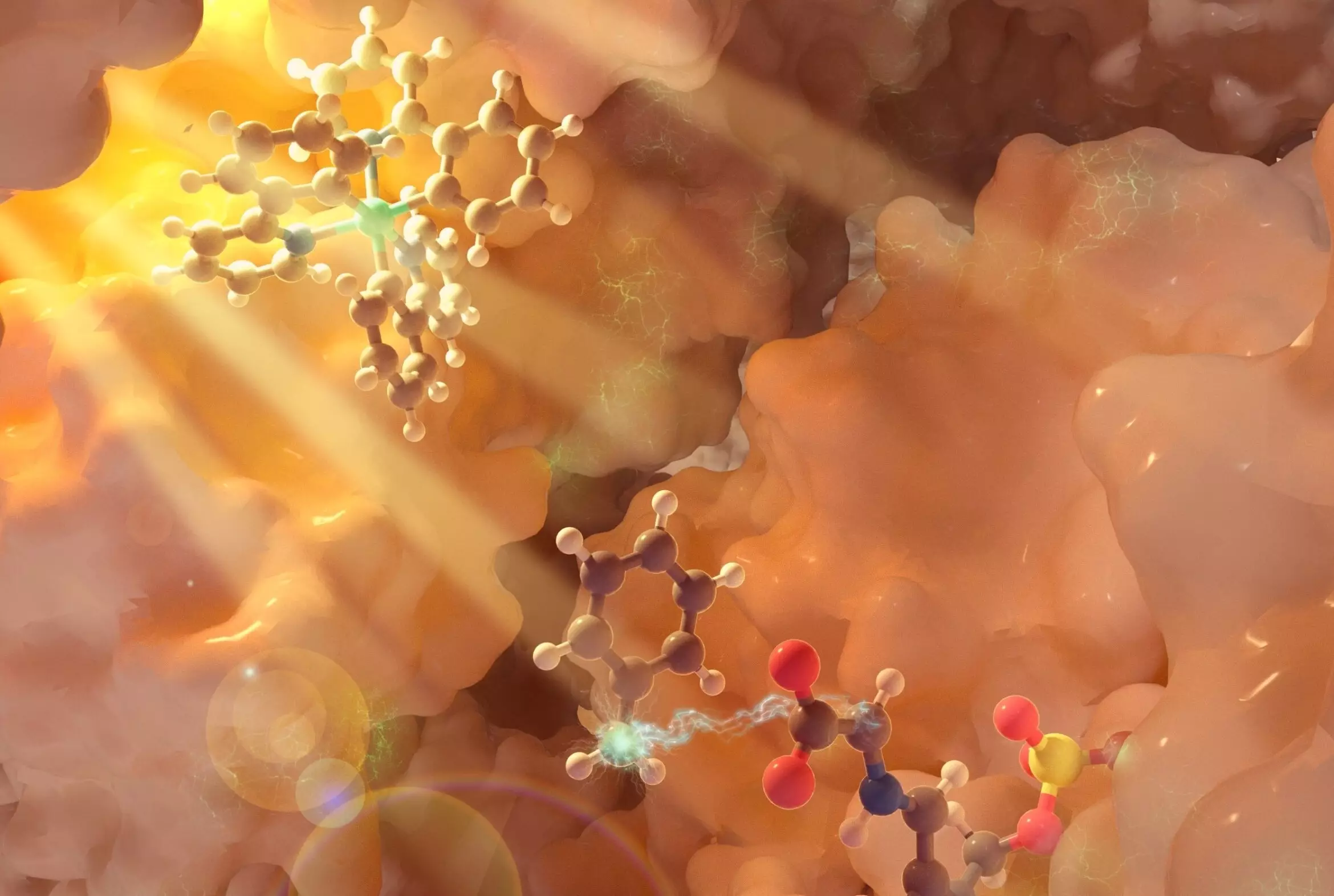
In their study, the research team focused on pyridoxal-phosphate (PLP)-dependent enzymes, a diverse group of catalysts involved in amino acid metabolism. Through a synergistic triple catalytic cycle, the researchers were able to harness the power of a photocatalyst, triggered by light, to initiate a chain of reactions that ultimately led to the modification of amino acid substrates. This intricate dance between photochemistry and biocatalysis enabled the creation of non-canonical amino acids with altered molecular structures, paving the way for the design of novel functional molecules.
One of the key breakthroughs of this study was the demonstration of radical-mediated alpha functionalization of common amino acid substrates using PLP enzymes. By forming new carbon-carbon bonds at the alpha carbon position of amino acids, researchers were able to introduce a plethora of new functionalities and features to these molecules. This innovative approach not only expands the chemical toolbox for designing therapeutics and natural products but also streamlines the synthetic process by eliminating the need for protective groups.
Furthermore, the photobiocatalytic method developed by the research team exhibited high stereoselectivity, meaning it could selectively produce amino acids with preferred three-dimensional shapes. This precise control over the stereochemistry of the products is essential for designing molecules with specific biological activities and properties. Additionally, the process proved to be highly efficient, offering a sustainable and environmentally friendly route to accessing valuable non-canonical amino acids.
As the research continues to unravel the intricacies of enzyme-photocatalyst interactions, there is a growing potential to enhance the synergy between these two catalysts and unlock even more complex chemical transformations. By pushing the boundaries of biocatalysis and incorporating light-driven processes, scientists are poised to revolutionize the way we approach synthetic chemistry and drug discovery. The future holds exciting possibilities for developing novel biomolecules with tailored functionalities and applications, all thanks to the power of light-driven biocatalysis.

Leave a Reply