The field of physics offers invaluable insights into the interactions between disparate substances, especially when investigating mixtures. Classical mixture theory provides a robust framework for modeling systems with two or more constituents, taking into account not only the proportion of each component but also the intricate interactions that can emerge. These principles are not exclusive to inanimate systems, as ongoing research indicates they yield significant implications in biological processes, particularly in understanding cellular structures. At São Paulo State University (UNESP), a team of researchers has taken the principles of condensed matter physics and applied them to cellular biology by examining protein compartmentalization, revealing parallels to the established concept of Griffiths phases seen in magnetic materials.
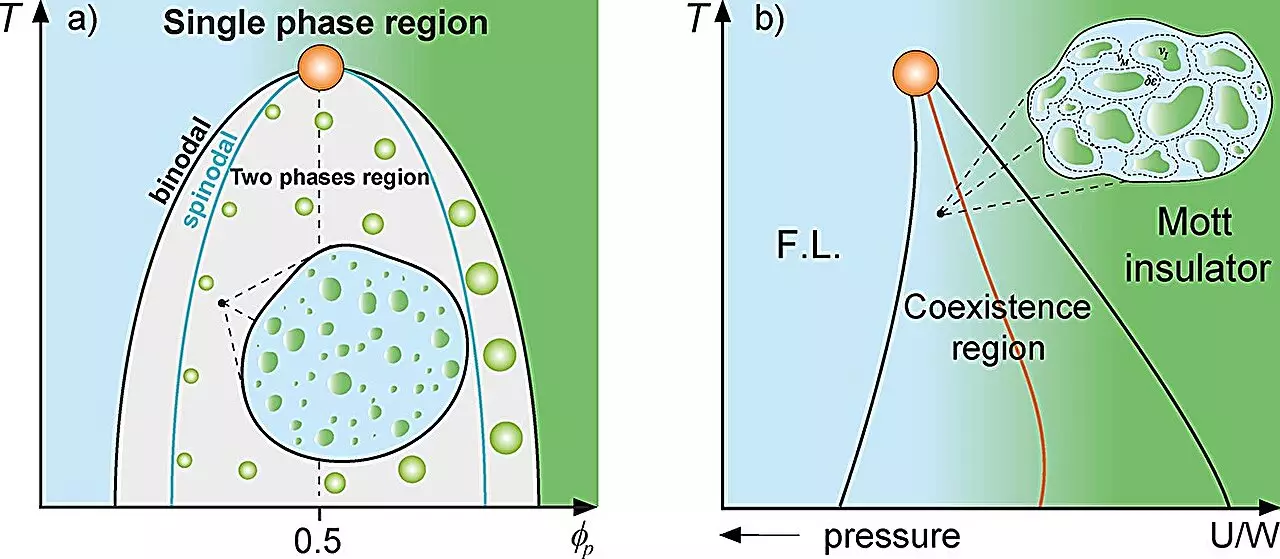
The magnetic Griffiths phase represents a fascinating interplay of magnetized and non-magnetized regions existing within a larger ferromagnetic or paramagnetic matrix. Rare regions appear spontaneously, affecting the overall dynamics and properties of the system. Lucas Squillante—a Ph.D. candidate—and Professor Mariano de Souza, both from UNESP, are exploring a similar phenomenon in the context of protein droplets that form within cellular environments. Their study draws an analogy between these protein-rich regions, dubbed ‘rare regions’ in the cellular milieu, and the magnetic Griffiths phase. Such insights enhance our comprehension of how proteins interact and become compartmentalized under specific conditions. This appropriation of physical principles illuminates the dynamics of life at a molecular level, offering new perspectives on how cellular organization arises.
The production of proteins within cells can reach critical levels that trigger liquid-liquid phase separation, resulting in the spontaneous formation of droplets. This phenomenon is not merely a random occurrence but can be analyzed through the lens of thermodynamics using models like the Grüneisen parameter and the Flory-Huggins model. The researchers found that cellular dynamics are markedly reduced near the phase separation threshold—a concept referred to as the binodal line. Such chains of biochemical events highlight how compartmentalization may serve specific functions critical for cellular tasks, such as optimizing gene expression. By framing cellular processes in terms of Griffiths-like phases, we gain clarity on how biological systems manage complex interactions and organization, dynamically responding to myriad environmental cues.
Delving further, the implications of these findings extend back to discussions surrounding the origins of life. Citing the pioneering theories of Aleksandr Oparin, the researchers contextualize their findings within the framework of primordial organisms. The survival of these early life forms may have depended on the liquid-like properties of coacervates—aggregates of organic compounds—that offered a conducive environment for biochemical reactions. Notably, homochirality emerges as a pivotal factor in the evolution of life, where microscopic chirality—exemplified by human hands—matters immensely. The study establishes a connection between increasing protein diffusion times within these droplet phases and reduced stochastic fluctuations, highlighting the codified balance essential for effective biological functionality.
Understanding protein compartmentalization not only enriches the field of biophysics but also holds significant implications in medical research, particularly concerning diseases. The literature extensively discusses how liquid-liquid phase separation relates to diseases—including cancer, neurodegenerative disorders, and eye diseases like cataracts—where aberrant protein aggregation may contribute to pathological states. The team proposes that the Griffiths-like cellular phase they identified can influence the pathology of these diseases by modulating protein behavior and interactions. As discussed by co-author Marcos Minicucci, this approach emphasizes the versatility of protein compartmentalization in pathophysiology, where the formation of protein droplets can have variable consequences—either aiding or exacerbating disease conditions.
The study conducted by the UNESP researchers exemplifies the power of interdisciplinary approaches in advancing fundamental scientific knowledge. By intertwining condensed matter physics with biology, they illuminate the intricate dance of molecular dynamics in living organisms. Their findings have the potential to reshape our understanding of cellular processes, with far-reaching implications for future research on health and disease. As our scientific community continues to bridge disparate fields, it uncovers innovative frameworks that will expand both our conceptual approaches and practical solutions in life sciences.

Leave a Reply