In an age where environmental concerns dominate public discourse, the need for sustainable and innovative chemical processes has never been more urgent. Traditionally viewed as a problem, carbon dioxide is now being reconsidered as a potential resource in the realm of synthetic organic chemistry. Researchers from various corners of the globe are pulling together their expertise to unlock the doors to efficient methanol synthesis, a liquid fuel that holds significant promise for energy alternatives. Through an exciting study published in *Nature Catalysis*, these scientists have unveiled groundbreaking techniques that not only utilize CO2 but do so with remarkable efficiency.
The Role of Advanced Materials
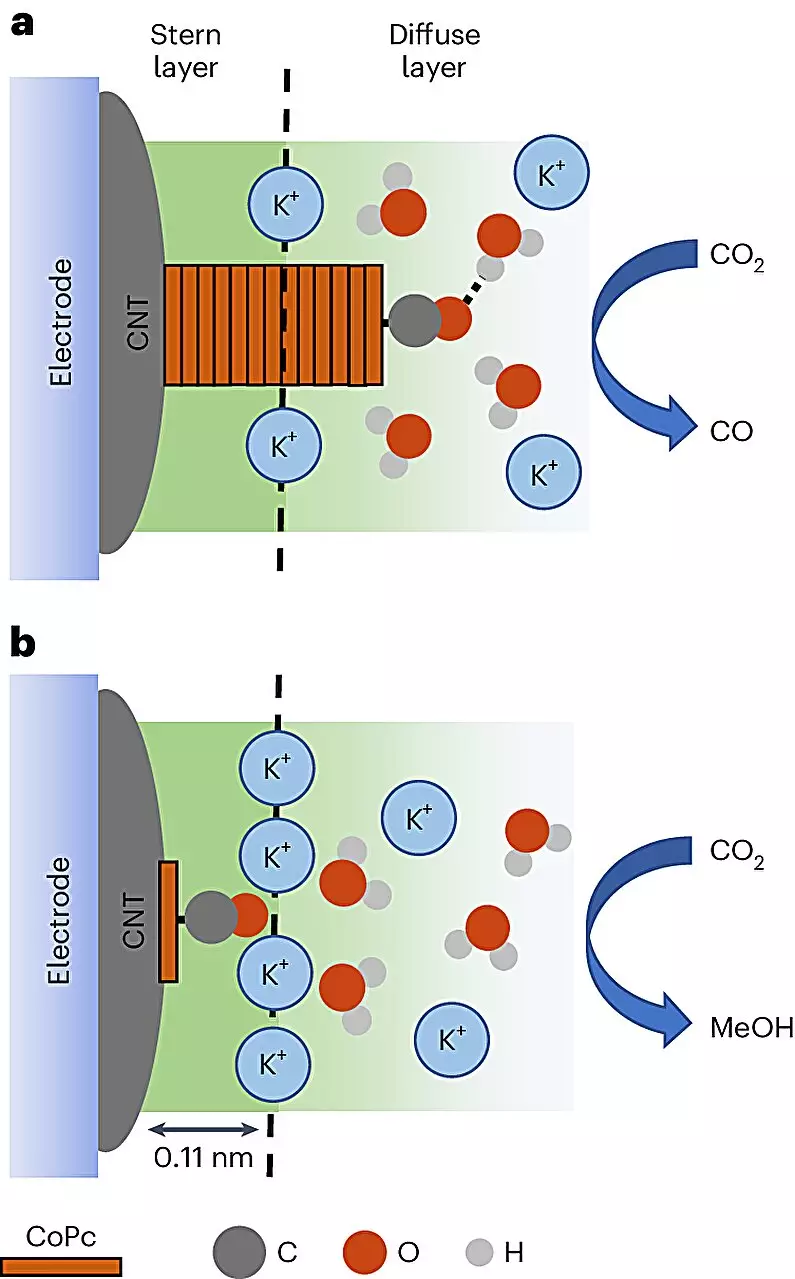
Central to this innovation is the employment of cobalt phthalocyanine (CoPc) molecules, which are deftly interfaced with carbon nanotubes—remarkable materials that boast exceptional electrical properties. These nanotubes serve as a playground for the CoPc molecules that, when submerged in an electrolyte solution and subjected to an electrical current, can engage in a fascinating transformation of CO2 into methanol. This process is important because it signifies a practical step in reducing greenhouse gas emissions while repurposing them into useful energy sources.
The integration of carbon nanotubes provides a foundational layer where electronics and chemistry converge, enabling the fine-tuning of reactions. One remarkable finding from the study reveals that by manipulating the catalytic surface environment, researchers could enhance methanol production rates up to eight-fold. This revelation is not just a minor increment; it illustrates a potential paradigm shift in how we approach the synthesis of fuels from waste. Robert Baker, co-author of the study, asserts that methanol’s high energy density makes it a desirable alternative fuel, and it’s clear that this research paves the way for its broader application.
Illuminating the Reaction Mechanism
The artistry of this study lies not only in the outcomes but also in the intricate methods used to analyze the reactions at play. For the first time, scientists have employed advanced in-situ vibrational spectroscopy to visualize the dynamics of catalyst interaction—an essential window into the previously opaque world of molecular transformation. Through this technique, researchers can observe the distinct behaviors of the CoPc molecules depending on their surrounding atomic environment, allowing for a real-time understanding of which conditions favor methanol production over less desirable by-products like carbon monoxide.
Quansong Zhu, the lead author of the study, elaborates on how these vibrational signatures reveal critical insights into reaction environments. By unearthing these relationships, the scientists are not merely documenting what happens; they are constructing a framework that could inform future methodologies for catalysis—a field long plagued by empirical trial-and-error tactics devoid of deeper understanding.
Cations: The Catalysts of Change
Moreover, the investigation dives deeper into the phenomenon of cations—charged particles that facilitate the catalysis process. Preliminary findings indicate that these supercharged entities are interacting directly with the CoPc molecules, thus amplifying methanol formation. Understanding the mechanics of these interactions opens the door for finer control over catalytic efficiencies, marking a significant step toward optimizing reactions for commercial viability.
As scientists continue to probe this front, the implications extend beyond mere academic enthusiasm. The possibility of generating methanol from renewable electricity positions it as a low-cost fuel alternative for significant sectors, including transportation, heating, and energy generation. This versatility underscores a transformative potential in both energy and chemical industries, showcasing the interlinked nature of modern scientific inquiry and real-world applications.
Anticipating Future Breakthroughs
With the groundwork laid, the researchers express enthusiasm for the potential applications arising from this study. The newfound insights are not just revolutionary; they signal a substantial leap toward more sustainable practices that prioritize resource-conversion processes over waste accumulation. Baker’s belief in the importance of understanding molecular interactions to enhance catalytic systems encapsulates the essence of scientific progress.
By focusing on waste molecule conversion technologies, we might be on the cusp of a new breakthrough era in renewable energy and materials science. The findings of this research could lead us to refine existing chemical processes further and inspire future discoveries. As chemists continue to unravel the complexities of molecular behavior while maximizing efficiency, one cannot help but feel optimistic about the path ahead. The convergence of innovative materials and sustainable practices indeed heralds a future where waste is no longer detrimental but rather a source of wealth and opportunity for mankind.

Leave a Reply