The pharmaceutical industry has long faced challenges in the production of peptide-based medicines, critical components in the fight against a range of debilitating diseases, including cancer and diabetes. Peptides, which are short chains of amino acids, serve as vital ingredients in various therapeutic applications, from medications and vaccines to innovative nanomaterials. The existing methods for producing these valuable substances, however, are fraught with environmental and logistical obstacles. Traditional chemical synthesis processes generate significant waste and employ toxic chemicals, raising concerns about ecological and health impacts. Now, scientists from the University of Manchester have unveiled an innovative approach that promises to change the landscape of peptide production, posing a substantial advancement over conventional practices.
The Breakthrough: Novel Ligase Enzymes
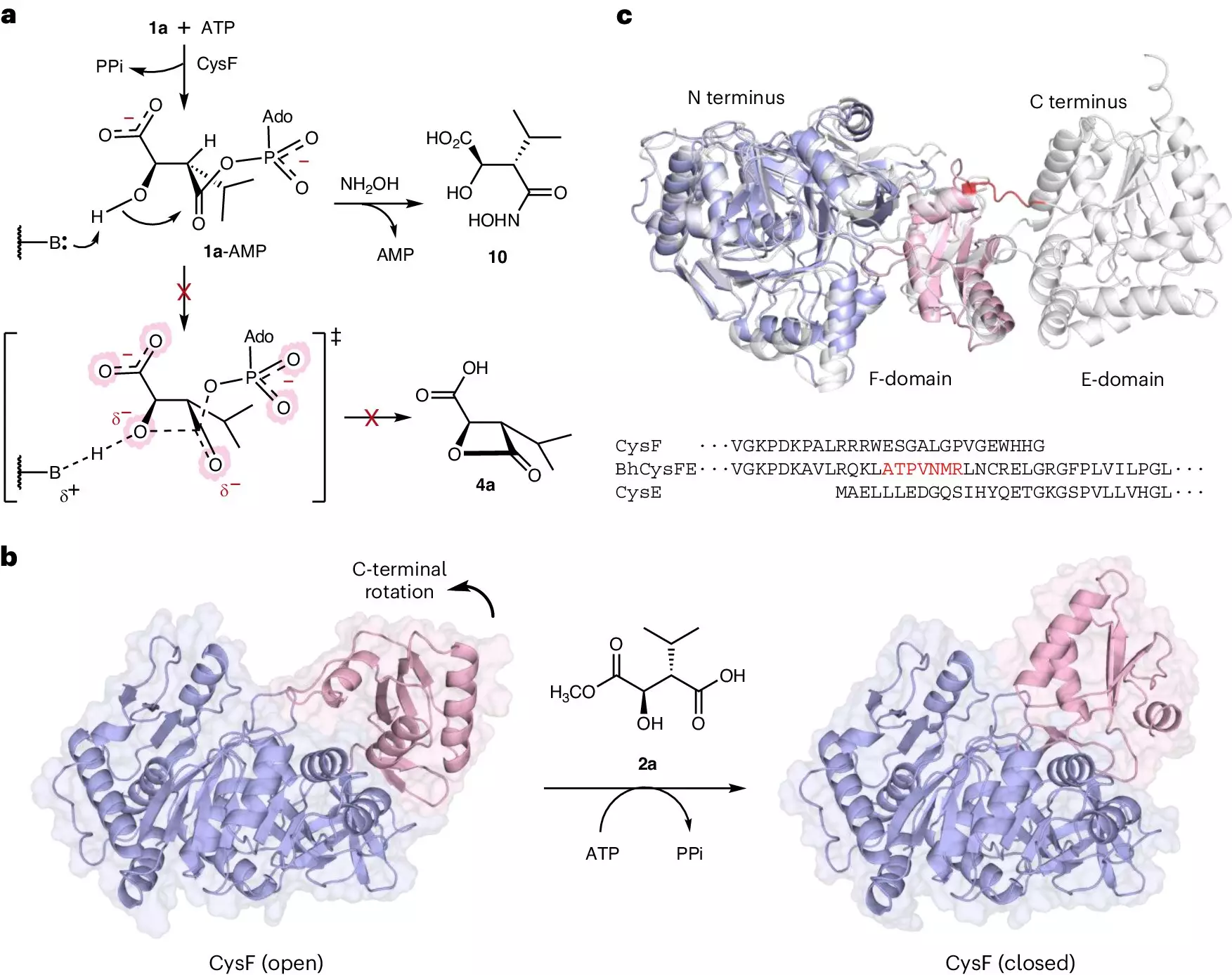
The breakthroughs presented in a recent publication in Nature Chemical Biology left the research community buzzing with excitement. The University of Manchester team discovered a new family of ligase enzymes—often described as molecular glue due to their role in facilitating the assembly of peptide sequences. This ingenious mechanism allows for simpler and more efficient production of peptides, substantially increasing yields compared to outdated methodologies. The lead researcher, Professor Jason Micklefield, emphasized this leap in efficiency, explaining how the combined use of different ligase enzymes enables a unified cascade reaction to produce various peptides in a streamlined way.
This new enzymatic technology could revolutionize the pharmaceutical production pipeline, particularly in the realm of oncology, where effective treatments are desperately needed. The simplification of peptide synthesis not only enhances the quantity of medicine produced but also significantly reduces harmful waste, aligning with global sustainability efforts that are increasingly vital in today’s medical landscape.
Challenges of Traditional Peptide Synthesis
Historically, the production of peptides has relied on a labyrinth of complex methods that involve multi-step chemical synthesis processes—often stretching across 10 to 12 laborious steps. These conventional techniques typically require heavily protected amino acid precursors and yield hazardous by-products that contribute to environmental degradation. Furthermore, high costs associated with raw materials and labor can stifle advancements in peptide therapeutics, leaving many potentially life-saving treatments out of reach for both manufacturers and patients alike.
The work from the Manchester team marks a pivotal shift away from these cumbersome processes toward a cleaner, more efficient model. By tapping into the natural biological processes of specific bacteria, researchers were able to isolate and characterize ligases that facilitate the natural assembly of peptides, thus providing a viable alternative to chemical synthesis.
Future Prospects: A Collaborative Approach
The practical implications of this research are vast and encouraging. Given that peptide therapies constitute a burgeoning sector of the pharmaceutical industry, the use of ligase enzymes could substantially reduce production times and costs across the board. The researchers are not resting on their laurels; they’ve initiated collaborations with leading pharmaceutical companies to optimize the output of these ligase technologies for widespread application. By refining the process for large-scale production, they aim to set the stage for a new era of peptide therapeutics that are not only more accessible but also environmentally responsible.
Moreover, the discovery is an open invitation for further exploration; the researchers have identified numerous other ligase clusters that may be instrumental in additional peptide pathways, hinting at a treasure trove of potential therapeutic applications yet to be unlocked. Dr. Guangcai Xu, a pivotal contributor to the project, noted that a new frontier in peptide synthesis and analysis is on the horizon, showcasing the profound impact that this research could have on various fields beyond just oncology.
In a world where the challenges posed by health crises continue to grow, innovations such as these are not just welcome; they are essential. By shifting the focus from traditional, environmentally harmful practices to cleaner, enzymatically driven methods, scientists stand on the cusp of a significant breakthrough in medicine. As the field moves forward, the ramifications of this research could extend far beyond peptide therapeutics, laying down a foundation for future generations of medicines that prioritize both efficacy and sustainability.

Leave a Reply