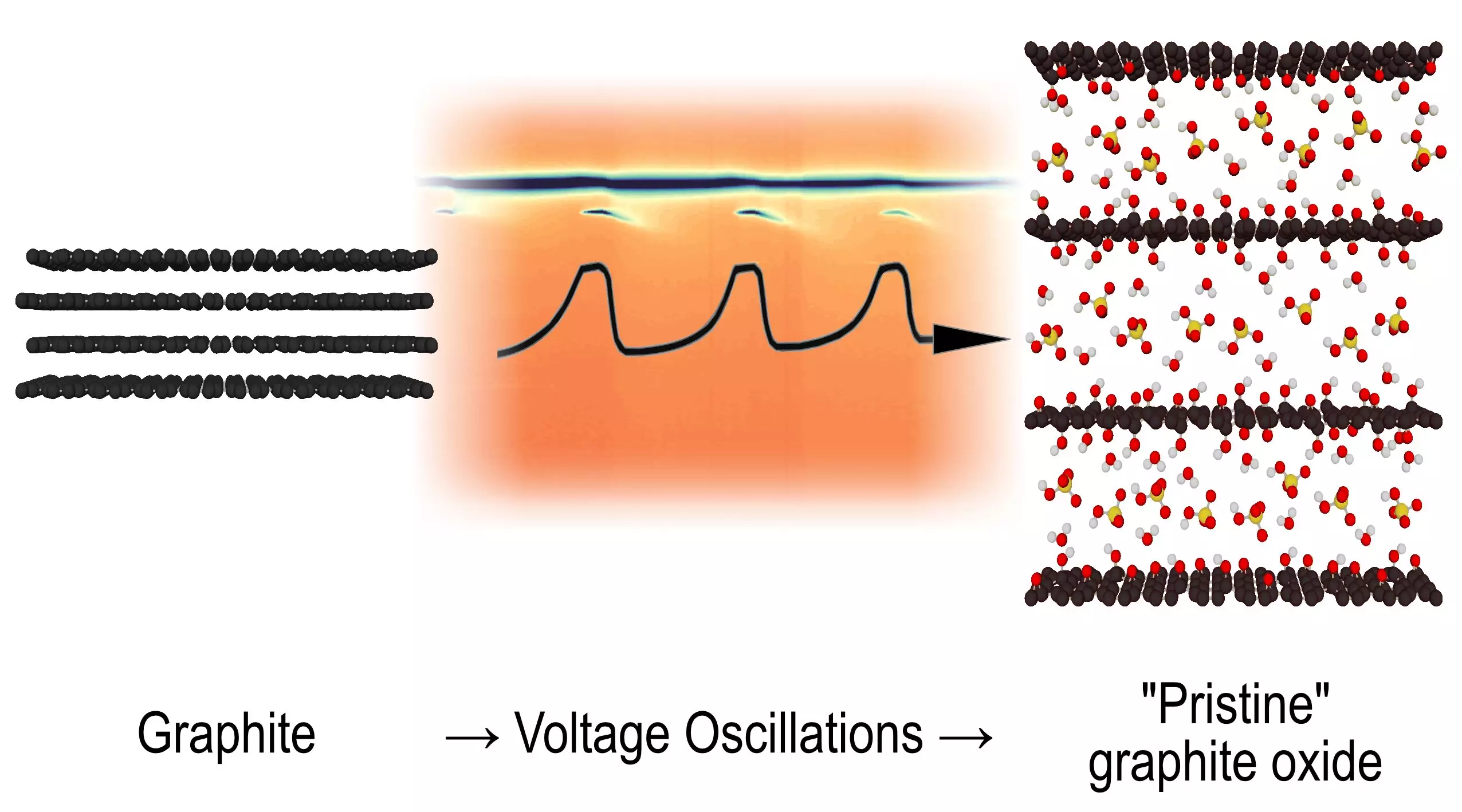
For decades, the intricacies of certain chemical reactions have left scientists scratching their heads, grappling with the enigmas of oscillating behaviors in systems that seemed to defy traditional chemical understanding. However, a team of researchers from Umeå University has recently cast light on a particularly puzzling reaction involving graphite and its transformation to graphene oxide, an event that has remained shrouded in mystery for over fifty years. Their findings, published in the renowned journal Angewandte Chemie, offer not only a resolution to a long-standing scientific riddle but also open up new avenues for inquiry into the fundamental principles governing chemical kinetics.
The reaction in question emerges when graphite is subjected to electrochemical oxidation within a sulfuric acid environment. While this process has been known to produce graphite oxide—the very material comprised of layered graphene oxide—the specific structural transformations that occur during the electrochemical oscillations had been poorly understood. Alexandr Talyzin, a notable professor in the Department of Physics at Umeå University, emphasized that while voltage oscillations during this process had long been documented, the underlying structural dynamics remained elusive. The team’s investigative zeal led them to utilize synchrotron radiation techniques, allowing them to capture rapid X-ray diffraction images, an approach that dramatically enhanced the temporal resolution of their observations.
The researchers were astonished to uncover intermediate structures that oscillate in a defined cycle. These transient formations materialize, vanish, and then reappear, demonstrating a dynamic interchange that challenges conventional perspectives on chemical reactions. The oscillation is not merely a simple back-and-forth motion; rather, it embodies a complex behavior that resembles what is commonly observed in living systems, where oscillatory patterns dictate various biological processes. The implications of this discovery are profound, as it blurs the lines between organic and inorganic chemistry. Talyzin pointed out that this oscillating reaction has previously been a subject of speculation within organic frameworks and now presents itself as an intriguing phenomenon in inorganic chemistry as well.
The realm of oscillating reactions gained significant recognition with Ilya Prigogine’s groundbreaking work, which earned him the Nobel Prize in 1977. His insights into non-equilibrium thermodynamics positioned oscillation as a fundamental mechanism where systems can transition from chaos to order. This latest discovery at Umeå University not only reinforces Prigogine’s theories but also suggests that there are more layers to explore. Talyzin’s team envisions that, if new theories emerge to explain their findings, this could spark significant advancements in the understanding of oscillating processes across various chemical systems. Such developments could yield novel applications in fields ranging from materials science to biochemistry.
The investigative process also included the creation of visually striking representations of the oscillatory behavior, culminating in a video that captures the rhythmic changes in the sample’s structure. This artistic representation not only serves as an educational tool to illustrate complex chemical principles but also emphasizes the intrinsic beauty of chemical dynamics—an aspect that is often overlooked in traditional scientific discourse. The elegance of oscillating reactions lies in their dual nature, embodying chaos and order in tandem.
As researchers delve deeper into the implications of this oscillating reaction, the horizon expands, presenting opportunities to uncover analogous phenomena and deepen our collective understanding of chemical reactions. The discovery at Umeå University marks a turning point, inviting chemists to rethink their understanding of chemical dynamics and challenging them to develop comprehensive theories that incorporate these newly observed oscillating behaviors. As these scientists embark on this exploratory journey, they will not only enrich the academic community’s knowledge but also potentially influence technological advancements in various fields.
The newfound understanding of oscillating reactions refines our comprehension of chemical processes, paving the way for future research. The vibrancy of this discovery illustrates the perpetual dance of molecules, one that holds the promise of transforming our understanding of science and its applications in the real world.

Leave a Reply