In a groundbreaking study led by Profs. Daniel Strasser and Roi Baer from The Hebrew University of Jerusalem, a team of international scientists has uncovered unforeseen symmetry-breaking dynamics in ionized carbon dioxide dimers. The research sheds light on the structural transformations that take place when these molecular clusters are exposed to extreme ultraviolet (EUV) radiation. Their findings, published in Nature Communications under the title “Symmetry-breaking dynamics of a photoionized carbon dioxide dimer,” offer new perspectives on molecular behavior in extreme conditions.
Redefining Quantum Mechanics Expectations
In typical environments such as outer space and atmospheric settings, carbon dioxide molecules usually form symmetric pairs. According to quantum mechanics principles, these molecular pairs should maintain their symmetry even after ionization. However, the team of researchers from The Hebrew University of Jerusalem, the Max Planck Institute for Nuclear Physics, and the FLASH free electron laser facility at DESY have witnessed a phenomenon known as symmetry-breaking. This unexpected behavior challenges established scientific models and theories.
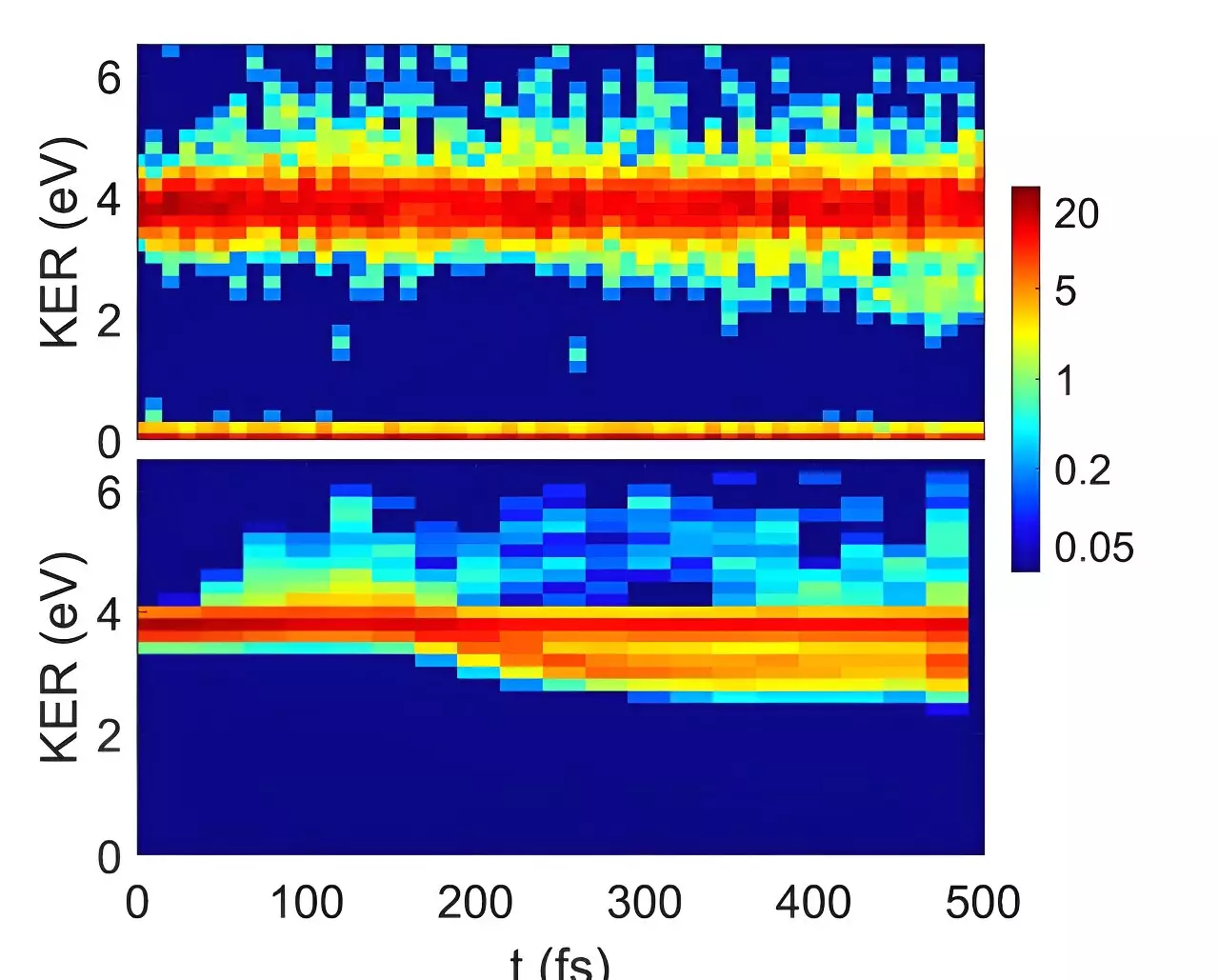
The study involved utilizing two quantum chemistry models to predict how ionized CO2 dimers would behave under extreme conditions. While one model anticipated the molecules to move together in sync, preserving their symmetry, the other model suggested that ionization would disrupt this symmetry. Surprisingly, the researchers were able to confirm that the ionized dimers indeed undergo asymmetric structural rearrangement, leading to the formation of CO3 moieties. This discovery has profound implications for atmospheric and astrochemistry, offering new insights into molecular processes in extreme environments.
One of the key questions raised by this study is how symmetry-breaking can occur despite quantum mechanics ostensibly prohibiting it. The researchers likened the carbon dioxide molecule pair to Schrödinger’s famous cat, existing in a superposition of two symmetry-breaking states. The system maintains symmetry until measurement collapses the quantum wave function, causing one of the CO2 molecules to rotate relative to the other. This unexpected behavior challenges conventional understanding of molecular dynamics.
Prof. Strasser emphasized the importance of these findings, noting that the combination of advanced experimental techniques and theoretical modeling can reveal unexpected molecular behavior. The insights gained from studying ionized carbon dioxide dimers could potentially revolutionize carbon dioxide chemistry and enhance our comprehension of planetary and atmospheric processes. Prof. Baer, who spearheaded the theoretical modeling, highlighted the importance of merging theory with experimental data to improve our ability to predict chemical reactions in remote environments and inaccessible laboratory settings.
The results of this study have far-reaching implications for atmospheric chemistry, astrochemistry, and our understanding of the atmospheric carbon dioxide cycle. The discovery of asymmetric structural rearrangements and the formation of CO3 moieties provide a deeper understanding of molecular processes in extreme conditions. By leveraging state-of-the-art facilities and international collaboration, the research team has opened up new avenues for investigating molecular clusters’ behavior under extreme conditions and exploring potential applications in atmospheric science and novel chemical synthesis methods. The innovative approach taken by the researchers could lead to further breakthroughs in the field of molecular physics.

Leave a Reply