Noble gases, long categorized as inert due to their reluctance to engage in chemical reactions, have puzzled scientists for decades. Traditionally, they were thought to be unreactive due to their complete valence electron shells. However, this notion was significantly challenged over 60 years ago when Neil Bartlett synthesized the first noble gas compound, xenon hexafluoroplatinate (XePtF6), awakening curiosity about the potential reactivity and utility of these elusive elements. The discovery not only marked a milestone in chemical history, earning Bartlett recognition as an International Historic Chemical Landmark but also opened the door to a variety of other noble gas compounds waiting to be explored.
The difficulties in studying noble gas compounds stem from their inherent instability, particularly in the presence of moisture. Many of these compounds are sensitive to air, rendering them challenging to synthesize and obtain clarity regarding their structures. Typical crystallization techniques yield small samples, often inadequate for comprehensive analysis using conventional single-crystal X-ray diffraction. As a result, researchers have long struggled to uncover the detailed geometries of noble gas structures, making many of these compounds shrouded in mystery.
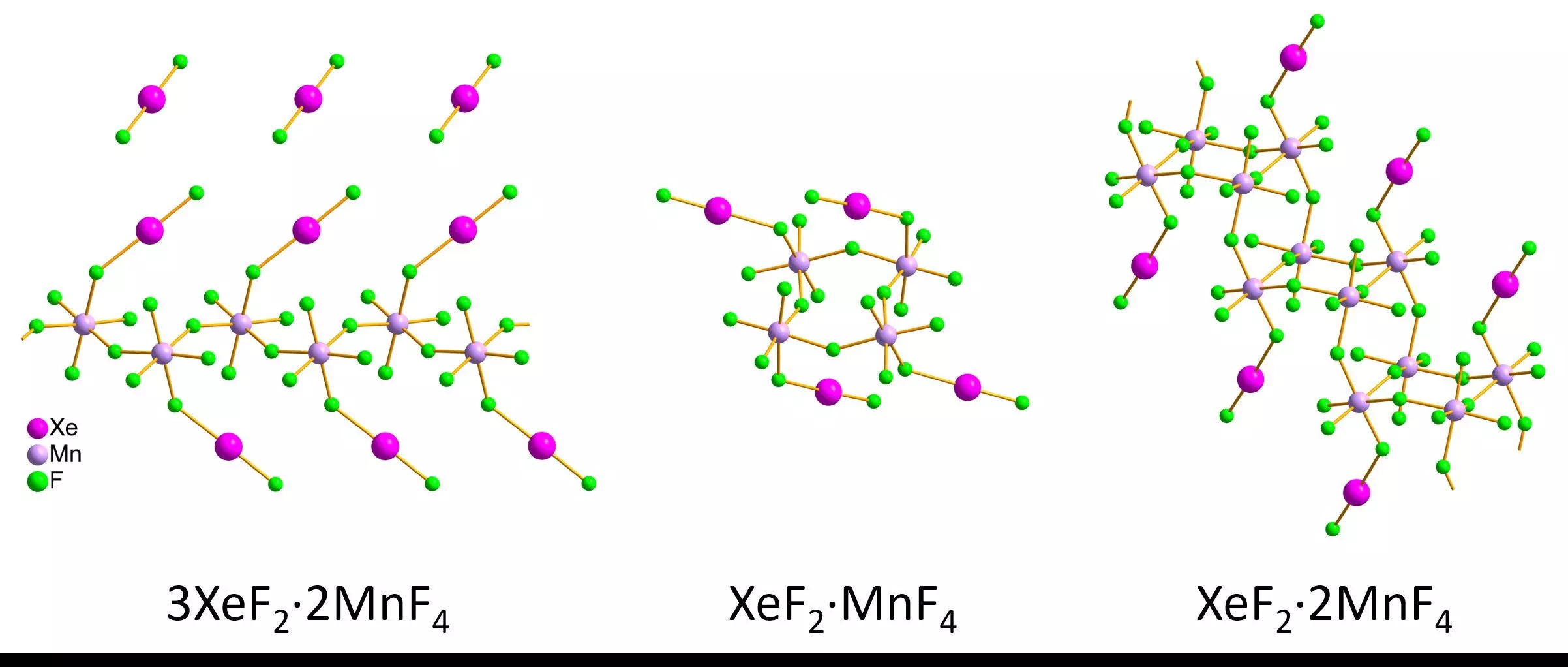
In a significant advancement, recent research has showcased the efficacy of 3D electron diffraction, a novel technique that allows scientists to analyze the structures of tiny crystallites, including those of air-sensitive noble gas compounds. Researchers led by Lukáš Palatinus and Matic Lozinšek embarked on an explorative journey to apply this method to several xenon-containing compounds. They successfully synthesized xenon difluoride-manganese tetrafluoride compounds, obtaining both red and pink crystals, and tested their structural integrity in carefully controlled environments. By employing liquid nitrogen to stabilize the samples during transfer to a transmission electron microscope, they ensured that the delicate crystallites remained intact.
The findings from the new 3D electron diffraction technique were promising. The researchers meticulously measured bond lengths and angles in nanoscale crystallites, comparing the results with those obtained from conventional methods on larger samples. The correlation between both techniques was striking, highlighting the reliability of 3D electron diffraction even when examining minute structures. Furthermore, the experimental results revealed diverse structural arrangements, including infinite zig-zag chains, rings, and staircase-like double chains across different xenon compounds. Such arrangements provide valuable insights into the bonding interactions within noble gases that were previously undecipherable.
The success of implementing 3D electron diffraction to unravel the structures of noble gas compounds heralds a promising new chapter in the field of inorganic chemistry. The potential to further explore challenging compounds like XePtF6 and other air-sensitive materials can significantly enhance our understanding of noble gases’ chemistry and their applications. This advancement not only reinforces the idea that even the most inert elements can display rich and fascinating chemistry but also inspires further research endeavors into the unknown territories of noble gas compounds. As we continue to innovate and refine our scientific methods, the implications for material science and chemistry could be profound, potentially leading to new developments in various industrial applications.

Leave a Reply