Cholera infections caused by Vibrio cholerae bacteria are known to be life-threatening, with the cholera toxin produced by the bacteria acting as the trigger for the severe symptoms. This toxin specifically binds to certain “sugar lipids” known as GM1 gangliosides on the surface of intestinal cells. This interaction between the toxin and GM1 gangliosides is one of the strongest known interactions between a protein and a sugar molecule, allowing the toxin to penetrate intestinal cells and lead to rapid loss of fluid.
In a groundbreaking interdisciplinary approach, a team comprising researchers from the University of Münster, ETH Zürich, and Leibniz Universität Hannover has delved into a crucial component of the GM1 cholera toxin complex. By utilizing a fluorinated GM1 analog, the team has uncovered key molecular mechanisms underlying the strong interaction between GM1 gangliosides and the cholera toxin for the first time. This in-depth analysis not only enhances our understanding of the disease but also opens up avenues for the development of novel drugs targeting cholera infections.
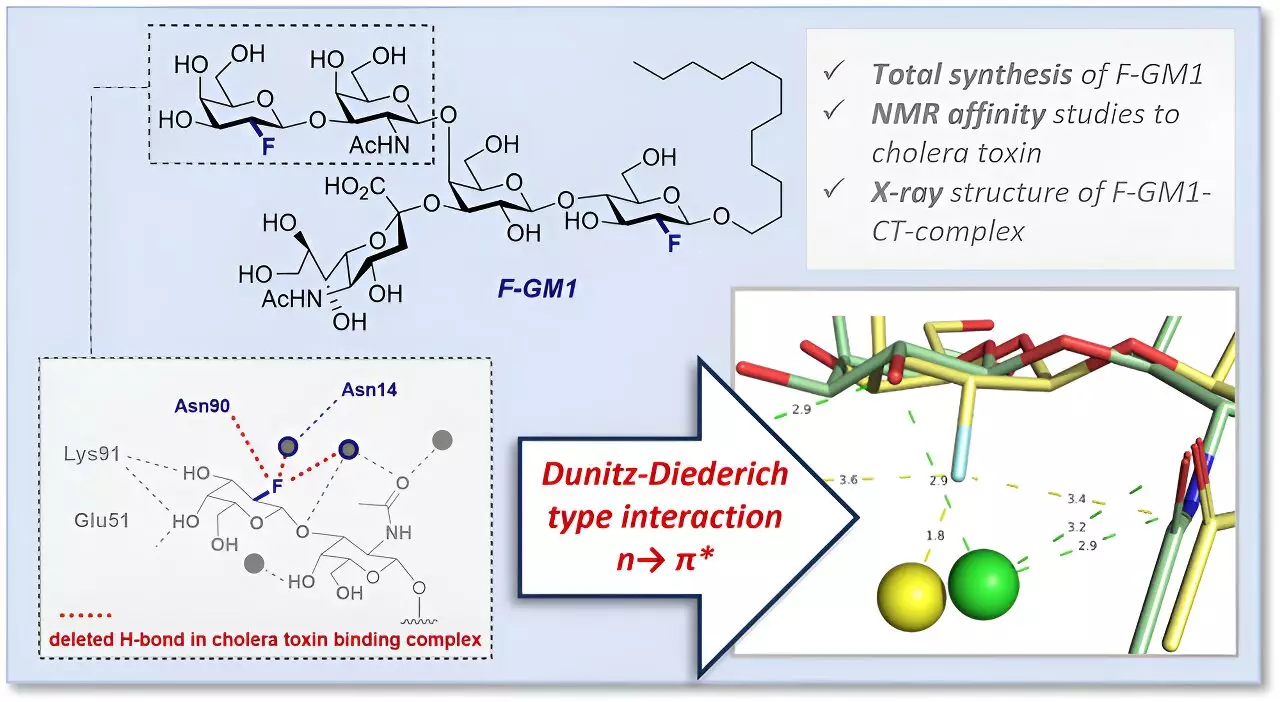
The team’s research involved the creation of a fluorinated GM1 analog (F-GM1) through a complex chemical synthesis process. By incorporating fluorine into the GM1 structure, researchers were able to discern the molecular interactions of this glycomimetic compound. Fluorine offered several advantages as a substitute for a hydroxyl group, including increased molecular stability against enzymatic degradation, precise control over spatial arrangement during glycosylation, and the ability to investigate interactions with the cholera toxin using nuclear magnetic resonance spectroscopy.
In addition to utilizing nuclear magnetic resonance spectroscopy, the research team employed protein crystallography to elucidate the interactions and spatial arrangement of atoms within the binding pocket of F-GM1. This detailed analysis allowed researchers to identify an additional interaction within the molecular complex induced by fluorine, as well as alterations in the arrangement of functional groups within the binding pocket. These findings shed light on the slightly lower affinity of F-GM1 for cholera toxin compared to natural GM1, providing valuable insights into the molecular basis of this interaction.
The study’s findings underscore the potential of utilizing fluorinated gangliosides in biomedical research, presenting opportunities to explore molecular signaling cascades involving sugar chains and identify novel therapeutic agents. By leveraging fluorine as a molecular tool, researchers can advance our understanding of complex cellular interactions and pave the way for innovative drug development strategies. The insights gained from this study may also have implications for vaccine development and other areas of infectious disease research.

Leave a Reply