Helices play a pivotal role in the world of biological molecules, particularly in proteins, influencing not only their structure but also their functionality. These spiral formations arise from the specific arrangement of amino acids, the fundamental building blocks of proteins. Each helix carries unique properties dictated by its sequence and structure, which ultimately dictate how proteins behave in various biological contexts. The intricate dance of these helical formations offers insights into cellular processes and has significant implications for biotechnology and medicine.
One of the most fascinating aspects of helices is chirality. Chirality refers to the property of a molecule that makes it nonsuperimposable on its mirror image, akin to left and right hands. In the formation of helical structures, chirality is crucial since it affects how amino acids are arranged and aligned, resulting in different functional properties for peptides. This relationship indicates that the orientation of these amino acids not only dictates the shape of the helix but also its interaction with other biological molecules, contributing to the overall functionality of proteins.
New Research Insights into Helical Structures
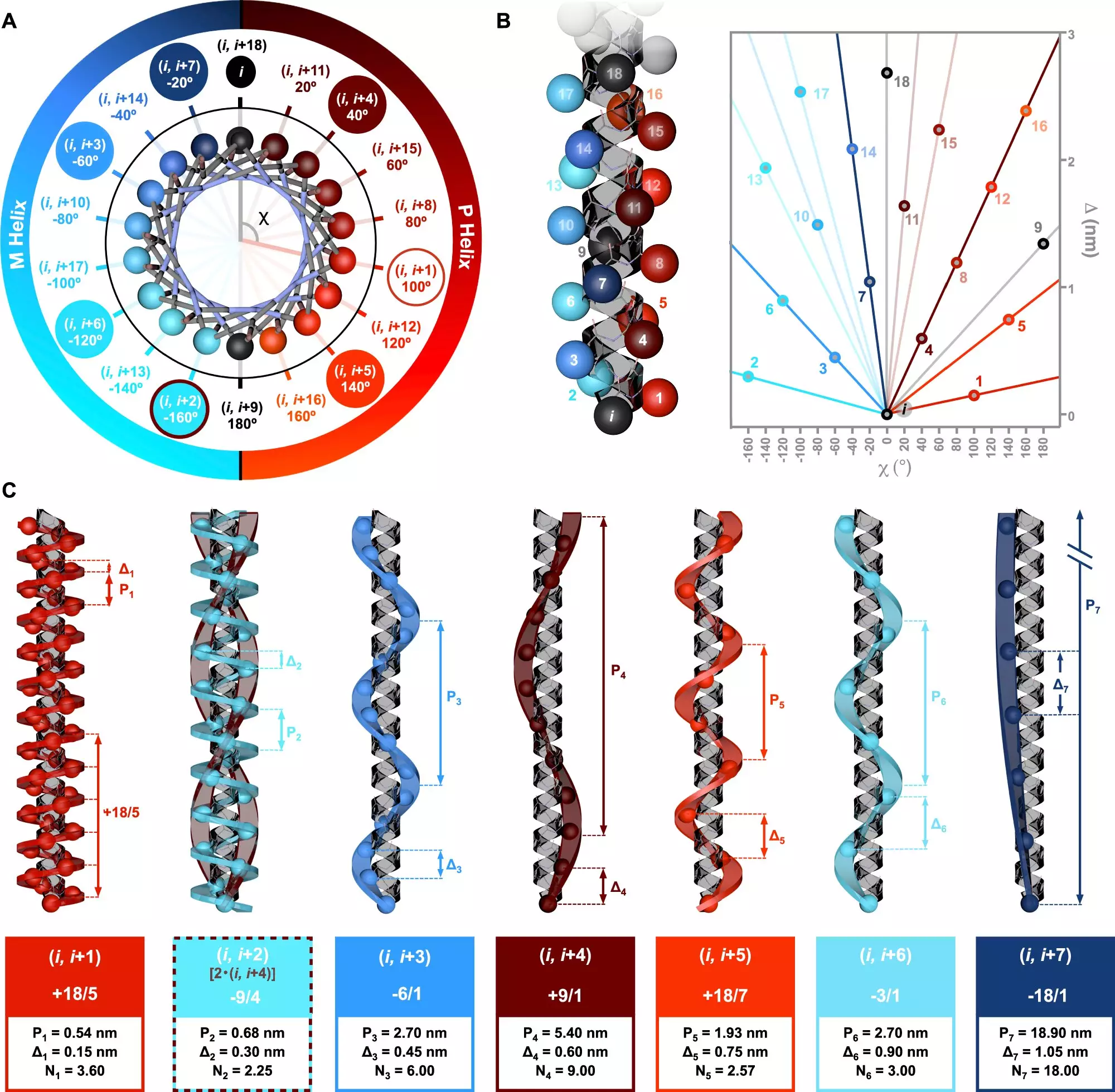
Recent research, spearheaded by Dr. Julián Bergueiro and his team at the Center for Research in Biological Chemistry and Molecular Materials (CiQUS), has unveiled groundbreaking insights into peptide helices. The study emphasizes the need for understanding the chiral characteristics of helices within peptide sequences. By employing innovative computational techniques along with circularly polarized light spectroscopy, the researchers were able to elucidate how various amino acid sequences contribute to the formation of helices. Their findings reveal a new symmetry model that significantly enhances our understanding of how these molecular structures relate to one another.
Implications for Protein Functionality
The implications of these findings extend beyond theoretical knowledge; they bear the potential to revolutionize our approach to designing molecules. According to Dr. Bergueiro, the study illustrates that altering the sequence of amino acids can lead to distinct helical structures, which in turn could influence the biological activity of proteins. Such understanding is crucial for developing novel applications in pharmaceutical design, where tailor-made peptides could improve drug efficacy and specificity.
The study heralds a new era in macromolecular engineering, where scientists can intentionally control the monomer sequences within polymers. This ability to design precise topologies allows for innovation in creating biocompatible materials and advanced biotechnology applications. By unlocking the secrets of helical arrangements and their chiral properties, researchers can harness these structures for a variety of applications, from targeted drug delivery systems to new biomaterials.
The exploration of helices within peptides opens up new avenues for scientific inquiry and practical application. As research continues to unfold, the understanding gained from these intricate structures may lead to significant advancements in the fields of biology, medicine, and materials science.

Leave a Reply