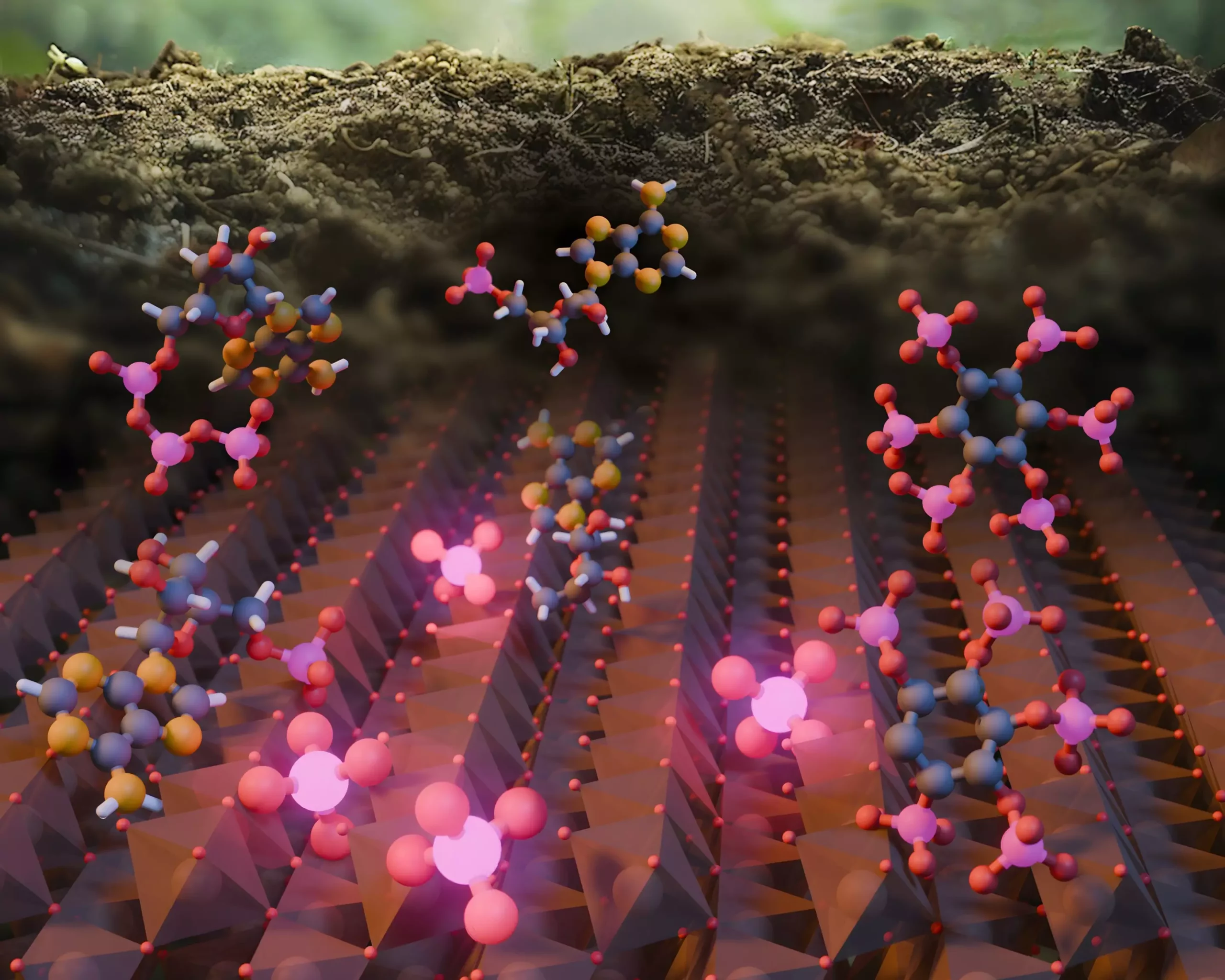
Recent research led by Northwestern University scientists has unveiled an astounding revelation regarding the cycling of phosphorus in nature—a nutrient essential for plant growth and integral to global food security. Published in Nature Communications, this study sheds light on a previously unrecognized mechanism that plays a significant role in the transformation of phosphorus, broadening our understanding of its cycle. Traditionally, the scientific community has attributed the transformation of organic phosphorus to inorganic forms primarily to enzymes produced by plants and microbes. However, this new study highlights the extraordinary capabilities of iron oxides, minerals commonly found in soils and sediments, to facilitate this vital process.
Phosphorus is a linchpin in the agricultural sector, indispensable for fertilizer production. As it stands, our reliance on mining for phosphorus sources poses risks, with estimates suggesting potential depletion within the next few decades. Therefore, comprehending how nature can recycle phosphorus more effectively is crucial for ensuring that future agricultural practices maintain robust crop yields.
The Role of Iron Oxides in Phosphorus Transformation
In exploring the phosphorus cycle, researchers led by Ludmilla Aristilde identified iron oxides as pivotal players in recycling this essential nutrient. The research team conducted intricate laboratory experiments, delving into the interactions between phosphorus and the minerals present in soils. Traditionally, the scientific narrative focused on biological processes as the driving force behind phosphorus cycling, but Aristilde’s compelling findings indicate that iron oxides may act as catalysts in this transformation. It is a groundbreaking assertion that challenges deep-rooted assumptions in environmental science.
Cleverly designed experiments revealed a remarkable ability of iron oxide minerals to enhance the conversion of organic phosphorus into bioavailable inorganic forms. Surprisingly, the rates at which these minerals recycled phosphorus matched those of biological processes, suggesting that nature employs a dual mechanism for this essential nutrient’s recycling—one anchored in biology and another rooted in mineral interactions.
Deciphering Nature’s Enzymatic Mix
The work raises profound questions about our understanding of ecosystem dynamics. Aristilde and her team used advanced X-ray techniques at the Stanford Synchrotron Radiation Lightsource to examine how phosphorus reacts in the presence of iron oxide minerals. They discovered that while some of the inorganic phosphorus generated was released into the solution, a significant portion remained affixed to the iron oxide’s surface. This unique finding implies that organic phosphorus can be effectively recycled from sources like DNA and RNA through non-biological means, further complicating the simplistic narrative that enzymes are solely responsible for this process.
The implications of these discoveries extend beyond mere academic curiosity; they challenge agricultural practices and open new pathways for enhancing food security. If we can leverage the natural mechanisms involving iron oxides, we could establish more sustainable phosphorus management strategies that mitigate the risks associated with depleting mineral resources.
Broader Implications for Earth and Beyond
The ramifications of understanding phosphorus cycling are vast, affecting global agriculture and ecosystem management. With the looming threat of phosphorus scarcity, integrating knowledge about both biological and mineral-mediated phosphorus recycling could empower sustainable agricultural practices. As researchers like Aristilde advocate for nature-based solutions, the findings urge policymakers and agricultural stakeholders to rethink their dependency on traditional fertilizer sources.
Moreover, the implications of this research may extend to planetary science. Aristilde’s insights about the behavior of iron oxides and phosphorus recycling offer intriguing avenues for understanding the potential for life on other planets, such as Mars. The prevalence of iron oxides on the Martian surface invites speculation about whether similar biochemical processes could occur there, leading to thoughtful discussions about extraterrestrial agriculture and the possibilities for colonization.
The Path Forward: Harnessing Nature’s Solutions
As the need for innovative solutions to nutrient cycling becomes increasingly pressing, the findings from Aristilde’s research underscore the necessity of a paradigm shift in how we approach agricultural sustainability. Understanding that minerals can substantially influence phosphorus recycling adds a critical layer to our comprehension of ecological processes and their integration into farming practices.
Investing in further research into mineral catalysts, alongside biological mechanisms, presents an opportunity to advance sustainable agricultural practices. Not only can this knowledge improve the resilience of food systems across the globe, but it can also promote more eco-friendly approaches to managing our finite natural resources. As scientists and agriculturalists work together, the potential to revolutionize nutrient recycling holds the promise of securing future food supplies in tandem with environmental stewardship.

Leave a Reply