In a groundbreaking development, chemists at the National University of Singapore (NUS) have pioneered the creation of hexavalent photocatalytic covalent organic frameworks (COFs) that mimic natural photosynthesis for the production of hydrogen peroxide (H2O2). Unlike the traditional method of H2O2 production which relies on costly noble metal catalysts and hazardous solvents, this innovative approach utilizes sunlight as an energy source, along with abundant water and air as feedstocks. This not only presents a more sustainable alternative but also offers a promising solution to the challenges faced by the conventional anthraquinone process.
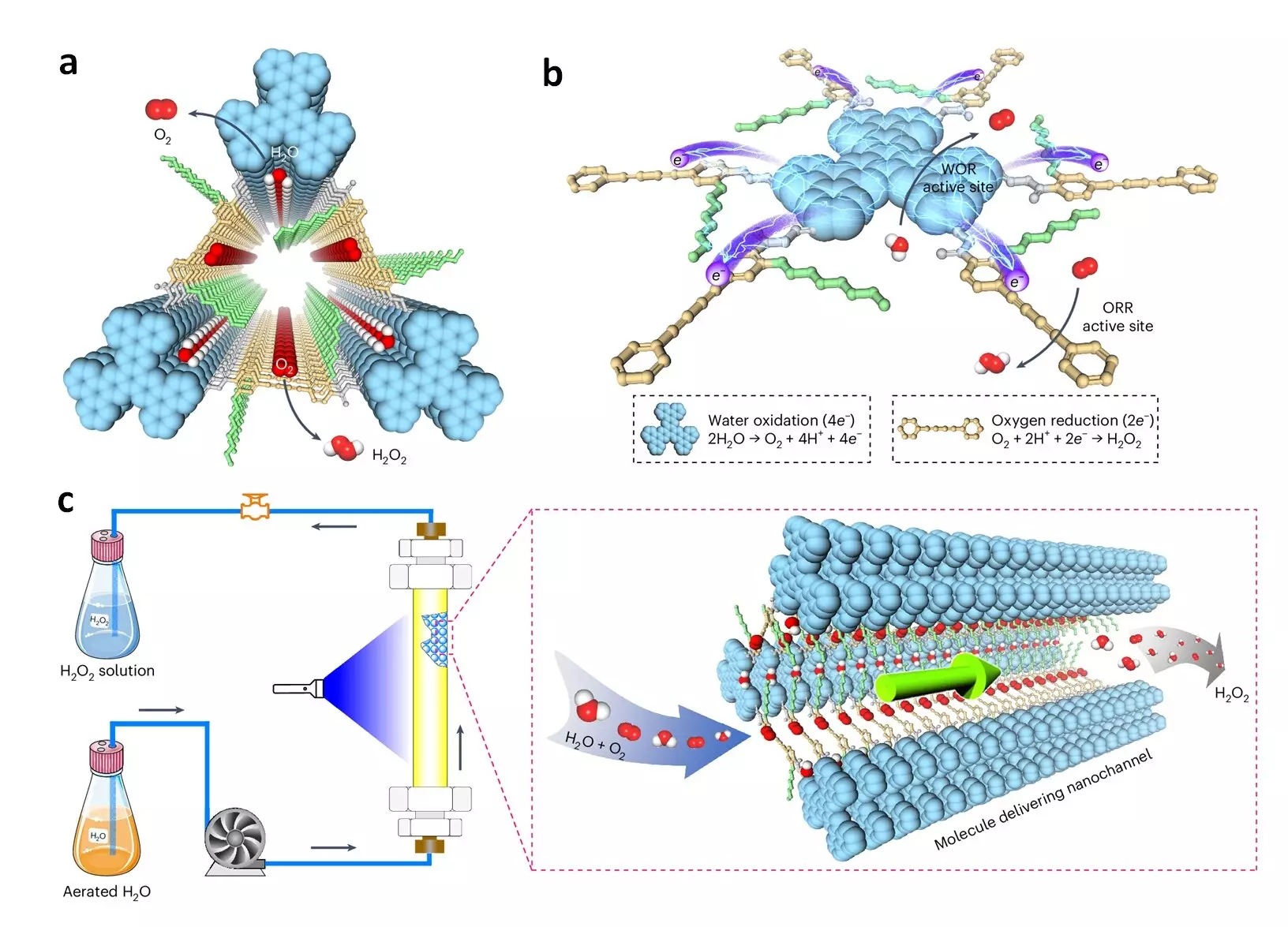
The artificial photosynthesis system faces three main challenges – insufficient charge carrier generation, limited catalytic sites, and inefficient delivery of charges and reactants to the catalytic sites. To address these challenges, the research team, under the leadership of Professor Donglin Jiang, has devised a novel approach. By developing hexavalent photocatalytic COFs through the systematic design of π skeletons and pores, the researchers have been able to overcome these obstacles. These porous, crystalline materials built from organic molecules linked by covalent bonds provide an ideal platform for constructing efficient photocatalysts.
The newly developed donor-alt-acceptor framework photocatalysts possess spatially segregated donor and acceptor columns for efficient separation of holes and electrons, preventing charge recombination and enabling rapid charge transport. Furthermore, the pore walls of the photocatalytic COFs are designed to be hydrophilic, facilitating the easy passage of water and dissolved oxygen to the catalytic sites through a 1-dimensional channel. This structural feature enhances the reaction kinetics and overall efficiency of the photocatalysts.
In terms of performance, the COFs demonstrate remarkable achievements. With a production rate of 7.2 mmol g–1 h–1, an optimal apparent quantum yield of 18.0%, and a solar-to-chemical conversion efficiency of 0.91% in bath reactors, these photocatalysts outperform traditional methods significantly. When integrated into flow reactors, they sustainably produce over 15 liters of pure H2O2 solution under ambient conditions, showcasing their operational stability over an extended period.
Prof. Jiang emphasized that this groundbreaking work represents the culmination of almost two decades of research in the field of COFs. By successfully addressing the challenges of delivering charges and reactants to catalytic sites efficiently, the development of these novel photocatalysts marks a significant advancement in the realm of sustainable hydrogen peroxide production. As the research findings are published in the esteemed journal Nature Synthesis, it is evident that the implications of this work extend beyond the laboratory and have the potential to revolutionize industrial processes worldwide.

Leave a Reply