The integration of small synthetic molecules inside protein crystals has shown great promise in the study of intermediate compounds formed during chemical reactions. Scientists from Tokyo Tech have reported on the success of this innovative method in visualizing reaction dynamics and rapid structural changes occurring within reaction centers. The research, published in Nature Communications, highlights the potential of this strategy in the intelligent design of drugs, catalysts, and functional materials.
The Challenges of Visualizing Short-Lived Intermediates
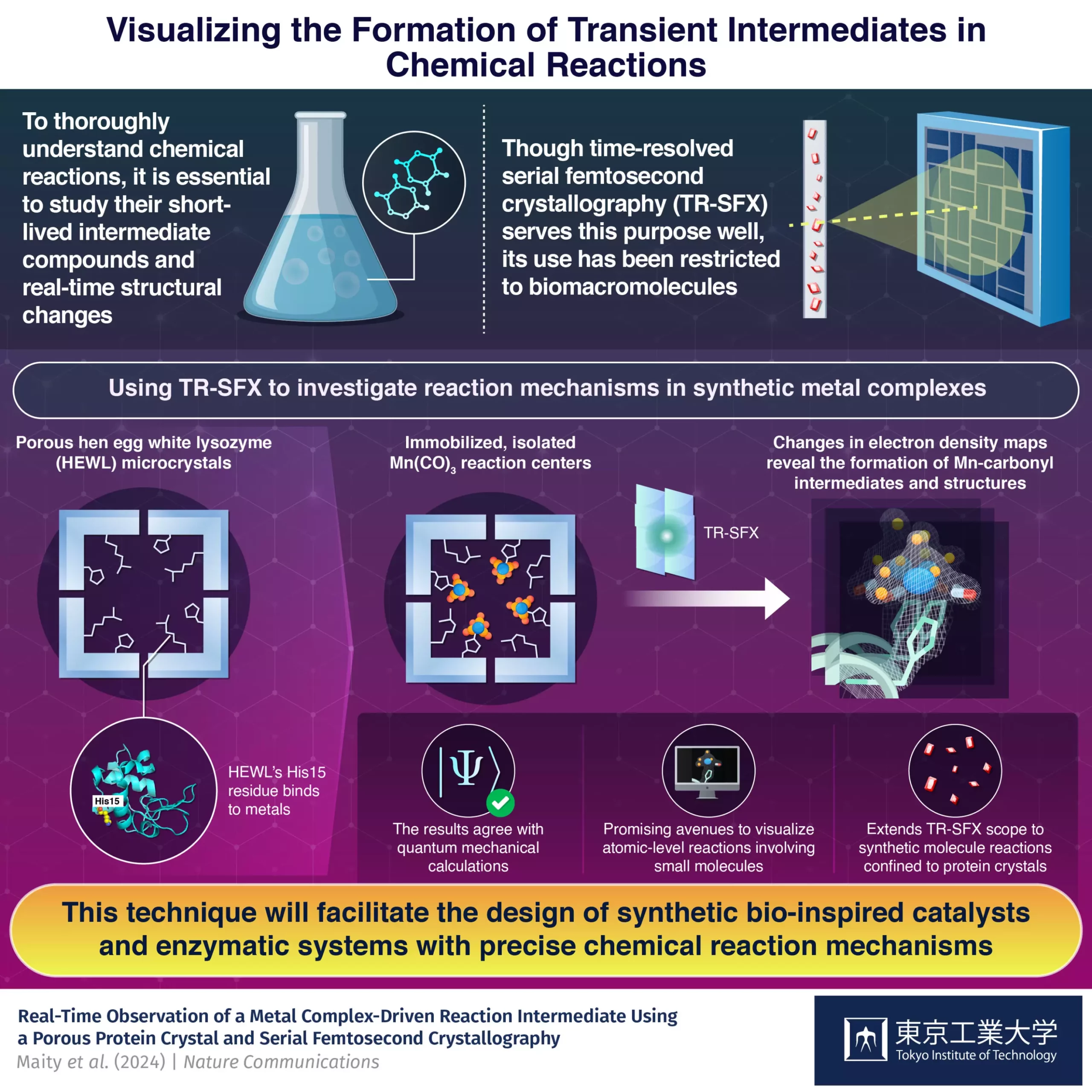
Most complex chemical reactions involve the formation of short-lived intermediate compounds before reaching the final products. Understanding these stepwise processes is crucial for advancements in various fields, including energy generation, catalysis, and medicine. However, capturing structural changes in molecules at the atomic level, especially in short-lived intermediates, is a challenging task. Time-resolved serial femtosecond crystallography (TR-SFX) has emerged as a cutting-edge method to visualize these processes by shooting fast electron laser pulses at crystallized molecular structures and capturing diffraction patterns.
While TR-SFX has primarily been limited to biomacromolecules, a research team led by Professor Takafumi Ueno from Japan decided to expand its applications to synthetic compounds. By immobilizing a light-sensitive Mn(CO)3-containing compound inside hen egg-white lysozyme (HEWL) crystals, the researchers were able to study the dynamics of carbon monoxide (CO) release. Overcoming limitations related to crystal size and packing density, the team successfully analyzed changes in electron density maps to understand the progression of the reaction.
Advancements in Studying Synthetic Metal Complexes
The innovative strategy developed by the research team enabled the isolation of Mn reaction centers within a protein environment, facilitating detailed experimental analysis of the intermediates generated during the reaction. By utilizing protein crystals as a matrix, the researchers were able to study the reactions of synthetic metal complexes with precision. The experimental results were found to be in excellent agreement with quantum mechanical calculations, validating the effectiveness of this approach.
The method of immobilizing small synthetic molecules inside protein crystals opens up new opportunities for studying chemical reactions involving non-biological components. This advancement holds the potential to revolutionize the design of artificial metalloenzymes and facilitate the development of innovative reactions. By investigating the mechanisms of reactions at the atomic level, researchers can enhance the design, control, and optimization of drugs, catalysts, and enzymatic systems.
The integration of small synthetic molecules inside protein crystals, coupled with time-resolved serial femtosecond crystallography, represents a significant advancement in the field of chemical reaction studies. The ability to visualize and analyze rapid structural changes and reaction dynamics at the atomic level provides valuable insights for the design of new drugs, catalysts, and functional materials. This innovative strategy paves the way for future research in understanding the intricacies of chemical reactions and developing tailored solutions for various applications in science and industry.

Leave a Reply