The atmosphere is a complex and intricate layer surrounding our planet, rich with a variety of chemical processes that govern life on Earth. Researchers continually uncover new compounds and reaction pathways that enhance our understanding of this vital system. Recent groundbreaking work from the Leibniz Institute for Tropospheric Research (TROPOS) in Leipzig has unveiled the existence of sulfurous acid (H2SO3) under atmospheric conditions—a finding that challenges long-held assumptions in atmospheric science.
Historically, the existence of sulfurous acid in an isolated gas phase has been regarded as nearly impossible. Studies have primarily concentrated on sulfuric acid (H2SO4), which is more commonly known and understood. Textbooks traditionally assert that H2SO3 could form in aqueous solutions of sulfur dioxide (SO2), yet attempts to detect this compound in such solutions have consistently failed. Since Helmut Schwarz’s team at TU Berlin first achieved the experimental detection of H2SO3 in 1988, using specialized mass spectrometry techniques, subsequent efforts have struggled to replicate this success. These challenges have led researchers to focus on its more stable counterparts, bisulfite (HSO3−) and sulfite (SO32−), severely constraining our understanding of sulfur chemistry in the atmosphere.
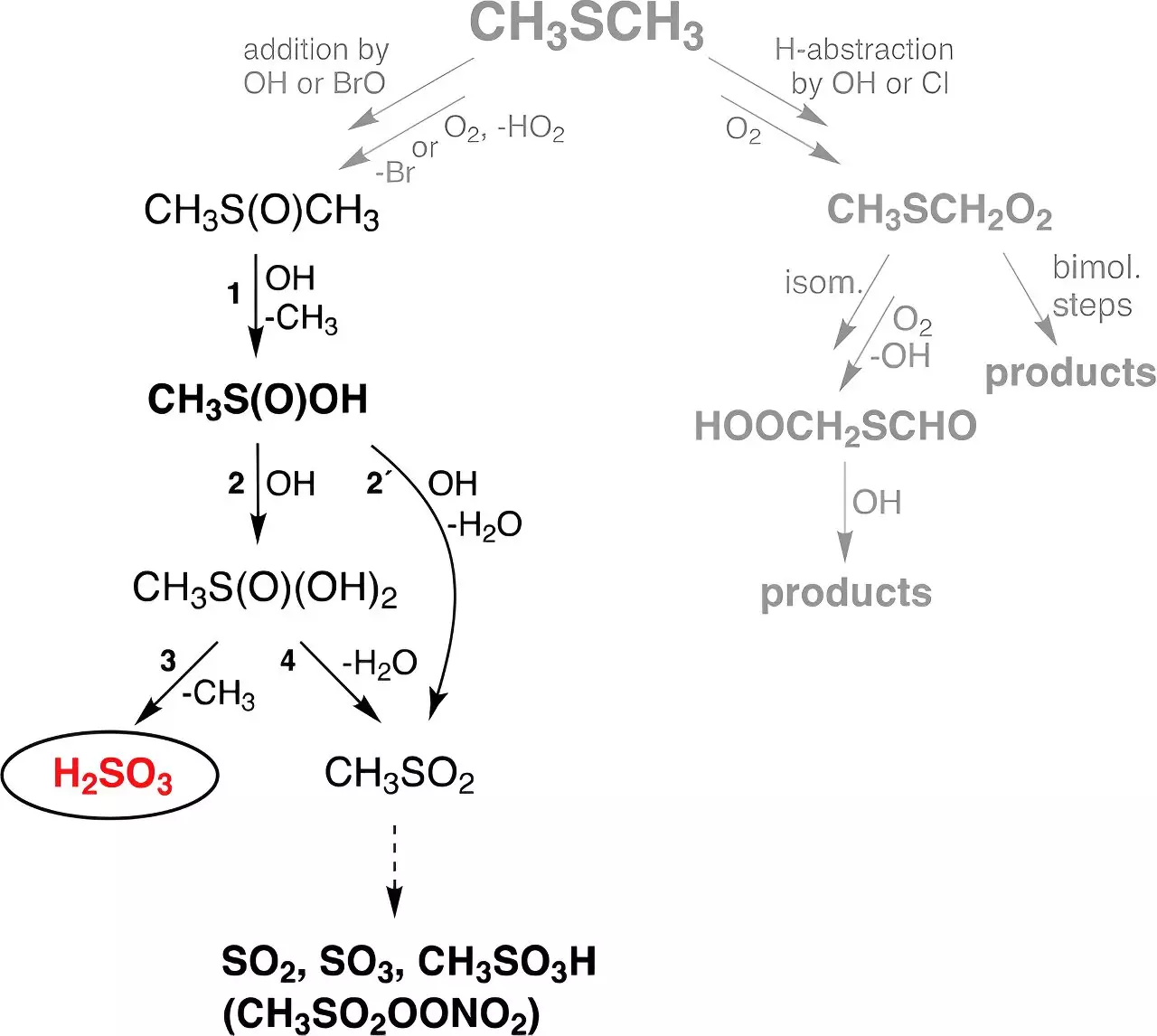
Emerging from these constraints, the recent work at TROPOS highlights a new pathway for the formation of H2SO3 through reactions involving dimethyl sulfide (DMS). DMS, a compound primarily produced in oceans by biological processes, serves as a substantial natural source of sulfur in the atmosphere. The researchers conducted a series of experimental investigations in flow reactors designed to simulate atmospheric conditions. Remarkably, they demonstrated that H2SO3 can indeed form and persist as a gas for a notable duration, remaining stable for about 30 seconds regardless of humidity levels. This revelation suggests the potential for H2SO3 to influence atmospheric chemistry more significantly than previously acknowledged.
Dr. Torsten Berndt and his team were particularly struck by the discovery, as the presence of H2SO3 in gas form had been assumed to be non-existent. The implications of this discovery extend beyond mere chemical curiosity; the research indicated that approximately eight million tons of H2SO3 could be produced globally each year from atmospheric reactions involving DMS. Furthermore, the findings suggest that this pathway generates an astonishing quantity of H2SO3—about 200 times that produced directly through the formation of sulfuric acid.
This increase in sulfurous compounds may play a critical role in the atmospheric sulfur cycle, potentially influencing various chemical reactions and impacts on climate. The study hints at a more complex interaction among atmospheric components than previously understood, inviting further investigation into the chemical dynamics at play.
Despite these groundbreaking findings, numerous questions remain unanswered. While the stability of H2SO3 in the gas phase has been established experimentally, researchers have yet to fully comprehend its lifetime regarding interactions with other atmospheric trace gases. The reaction of H2SO3 with water vapor is another area where further exploration is necessary. Dr. Berndt and his colleagues emphasize the need for optimized experimental methods to explore these interactions further, which could offer valuable insights into the role of H2SO3 in the larger context of atmospheric chemistry.
The challenges inherent in detecting and analyzing trace gases, especially compounds once deemed elusive, underscore the importance of advanced detection methods. Utilizing sophisticated mass spectrometry with an exceptional detection threshold allows for the observation of minute quantities of H2SO3, enabling researchers to glean deeper insights into atmospheric reactions.
The discovery of sulfurous acid in a gas form is a promising advancement for the field of atmospheric chemistry. As researchers delve deeper into the implications of H2SO3 and its impact on atmospheric dynamics, it is likely that this compound will generate significant interest for further study. Improved detection techniques combined with a deeper understanding of sulfur chemistry may pave the way for expanded knowledge of atmospheric processes, enriching our grasp of environmental science and its implications for climate. New insights into reaction pathways offer a glimpse into the intricate web of interactions within our atmosphere, fostering curiosity and driving progress in scientific exploration.

Leave a Reply