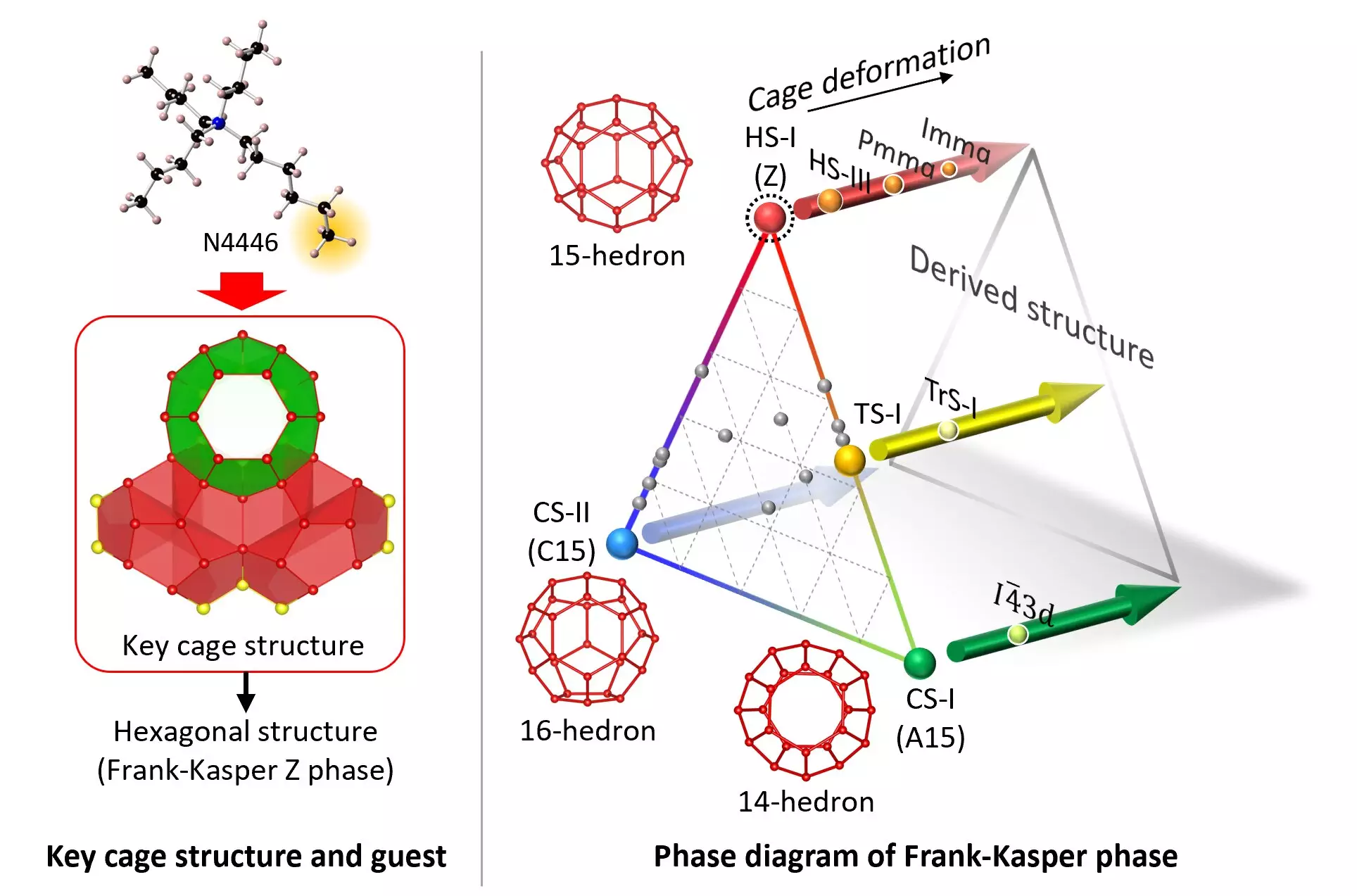
Clathrate hydrates are intricate water structures that encompass foreign molecules within a shell of water molecules. These structures play a significant role in material science research due to their unique properties and potential applications. Water molecules, consisting of two hydrogen atoms and one oxygen atom, have the ability to form weak bonds with each other and other molecules, altering their physicochemical characteristics. Clathrate hydrates are formed when water molecules assemble around guest substances, creating hydrogen-bonded frameworks with a close-packed tetrahedra arrangement.
The HS-I structure, a hexagonal crystal form of clathrate hydrate, has been challenging to stabilize in the past. However, a team of researchers from Yokohama National University and the National Institute of Advanced Industrial and Science Technology (AIST) in Japan successfully synthesized a stable form of HS-I clathrate hydrate. By fine-tuning the guest molecule, tri-n-butyl, n-hexylammonium chloride (N4446Cl), the researchers were able to create the necessary water-molecule cage structure for the HS-I form. This breakthrough opens up possibilities for engineering clathrate hydrates with tailored properties for various applications.
The discovery of stable HS-I clathrate hydrate under ambient temperature and pressure conditions is a significant advancement in the field of material science. Previous studies focused on extreme conditions to create new water structures, but this research showcases the ability to apply physicochemical properties to ecological research and simplify material development. The implications of synthesizing stable HS-I clathrate hydrate are vast, ranging from storage and transportation technologies for natural gas and synthetic fuels to carbon dioxide separation and recovery technologies. Additionally, this discovery may lead to the development of new materials incorporating mixed phases for a diverse array of applications.
Moving forward, researchers plan to explore the application of HS-I clathrate hydrate in various technologies, including natural gas storage, synthetic fuel transportation, and CO2 capture. By continuing to fine-tune guest molecule structures, the synthesis of additional FK phase structures for different compounds is likely. The development of new materials incorporating mixed phases holds promise for advancing material science exploration and creating innovative solutions for real-world challenges.
The synthesis of stable HS-I clathrate hydrate marks a significant milestone in material science research. This breakthrough opens doors to a wide range of applications and showcases the potential of harnessing water lattices for ecological research and material development. By understanding and utilizing the unique properties of clathrate hydrates, researchers can continue to make strides in creating functional materials with diverse applications.

Leave a Reply