The interaction between bodily carbohydrates, known as glycans, and key enzymes has been found to play a significant role in the development of various diseases. Research has shown that malfunctions in the attachment of glycans to proteins and lipids can lead to a heightened risk of conditions ranging from cancer to Alzheimer’s. A recent study, published in the Journal of Biological Chemistry, delves into the mechanisms by which enzymes interact with glycans, shedding light on the potential implications for disease pathogenesis.
Glycosylation, the process of attaching glycans to proteins or lipids, is crucial for numerous physiological processes within the body. From cell recognition to immune response, proper glycosylation is essential for the overall health and functioning of cells. Even minor changes in the structure of glycans can have a profound impact, contributing to the onset or progression of diseases such as diabetes and muscular dystrophy. This complexity has led to the emergence of glycobiology as a distinct field dedicated to studying the role of glycans in health and disease.
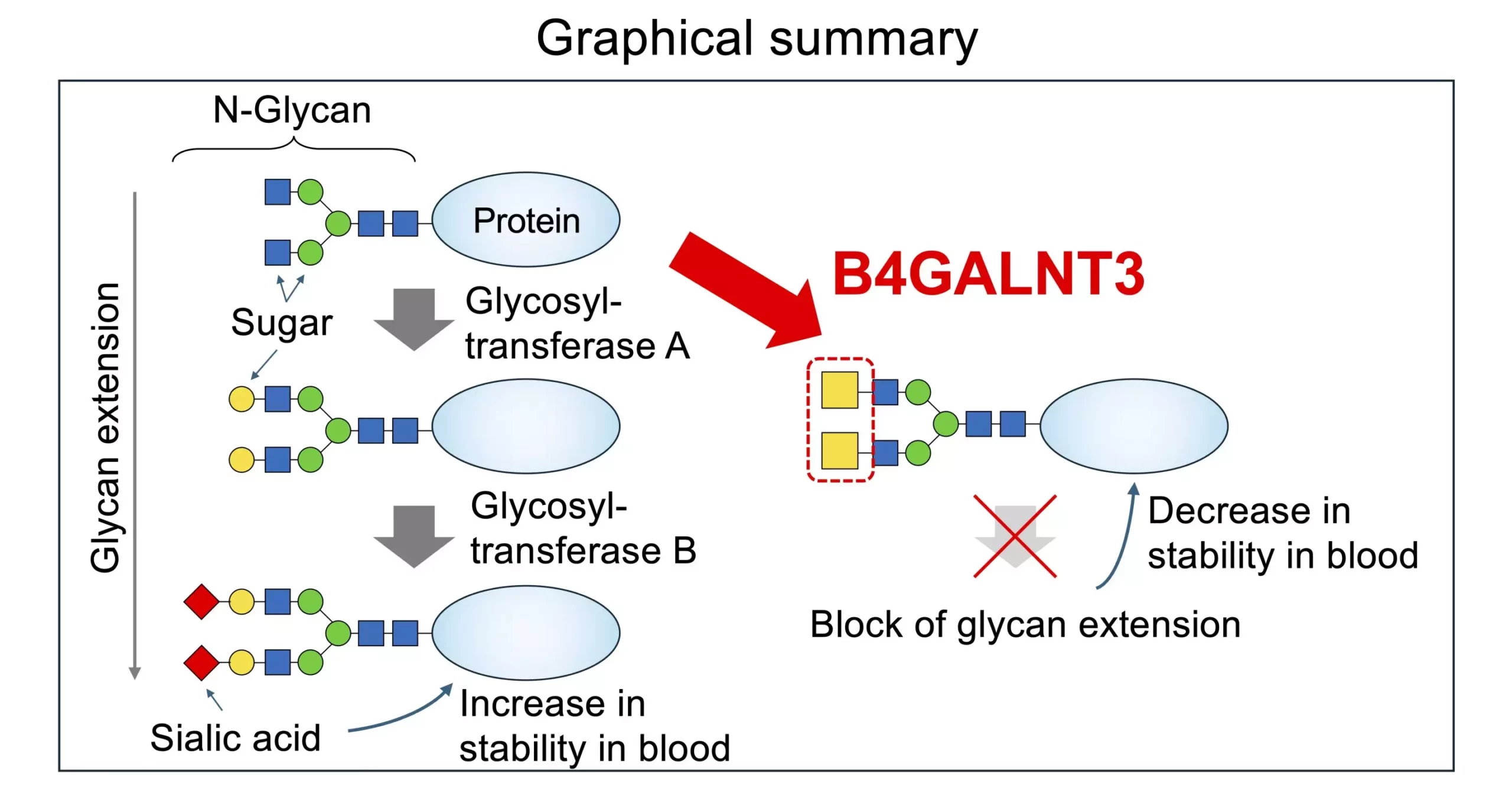
While much progress has been made in identifying the enzymes involved in glycan biosynthesis, there is still a need for a deeper understanding of how these enzymes are regulated within cells. One particular area of interest is the interaction between enzymes, known as glycotransferases, and a structure within glycans called LacdiNAc (LDN). This structure, found at the terminal or sub-terminal position of certain glycans, has been implicated in a variety of physiological processes and diseases.
Researchers have made significant strides in elucidating the role of the enzyme B4GALNT3 in catalyzing the biosynthesis of LDN. By examining the structural features of this enzyme and its unique “PA14 domain,” they were able to uncover the essential role of the PA14 domain in enzyme activity. Mutated versions of B4GALNT3 lacking the PA14 domain showed a marked decrease in activity related to glycan biosynthesis, highlighting the importance of this domain in regulating enzymatic function.
Implications for Disease Pathogenesis
The presence of LDN has been shown to have a significant impact on the production of N-glycans, influencing the modification of sugar residues and glycoproteins. This finding suggests that LDN plays a distinct role in shaping the structure and function of glycoproteins compared to the more common LacNAc structure. Understanding the specific effects of LDN on glycan biosynthesis could provide valuable insights into disease mechanisms and potential therapeutic targets.
Moving forward, researchers aim to further investigate the role of B4GALNT3 and LDN in disease pathogenesis, with the goal of identifying new therapeutic strategies. By leveraging advances in structural biology and enzyme analysis, they hope to unravel the complexities of glycan biosynthesis and enzyme regulation. Ultimately, this research has the potential to transform our understanding of how glycans and enzymes interact to impact disease progression and open up new avenues for intervention.

Leave a Reply