Chemistry has always been a field that requires precision and control over reactions. However, with the development of a new type of nitrene by a team of chemists at the University of Bremen, in Germany, the possibilities for slow reactions lasting up to three days have opened up new doors. This breakthrough could potentially revolutionize the way we approach chemical reactions in various applications.
Nitrenes, although reactive intermediates and analogs of carbenes, have always posed a challenge to chemists due to their brief reaction times, typically measured in nanoseconds. This limited window of time has made it difficult to utilize nitrenes in commercial applications effectively. The new slow-reacting nitrene developed by the researchers at the University of Bremen could overcome this obstacle and pave the way for a whole new class of nitrenes.
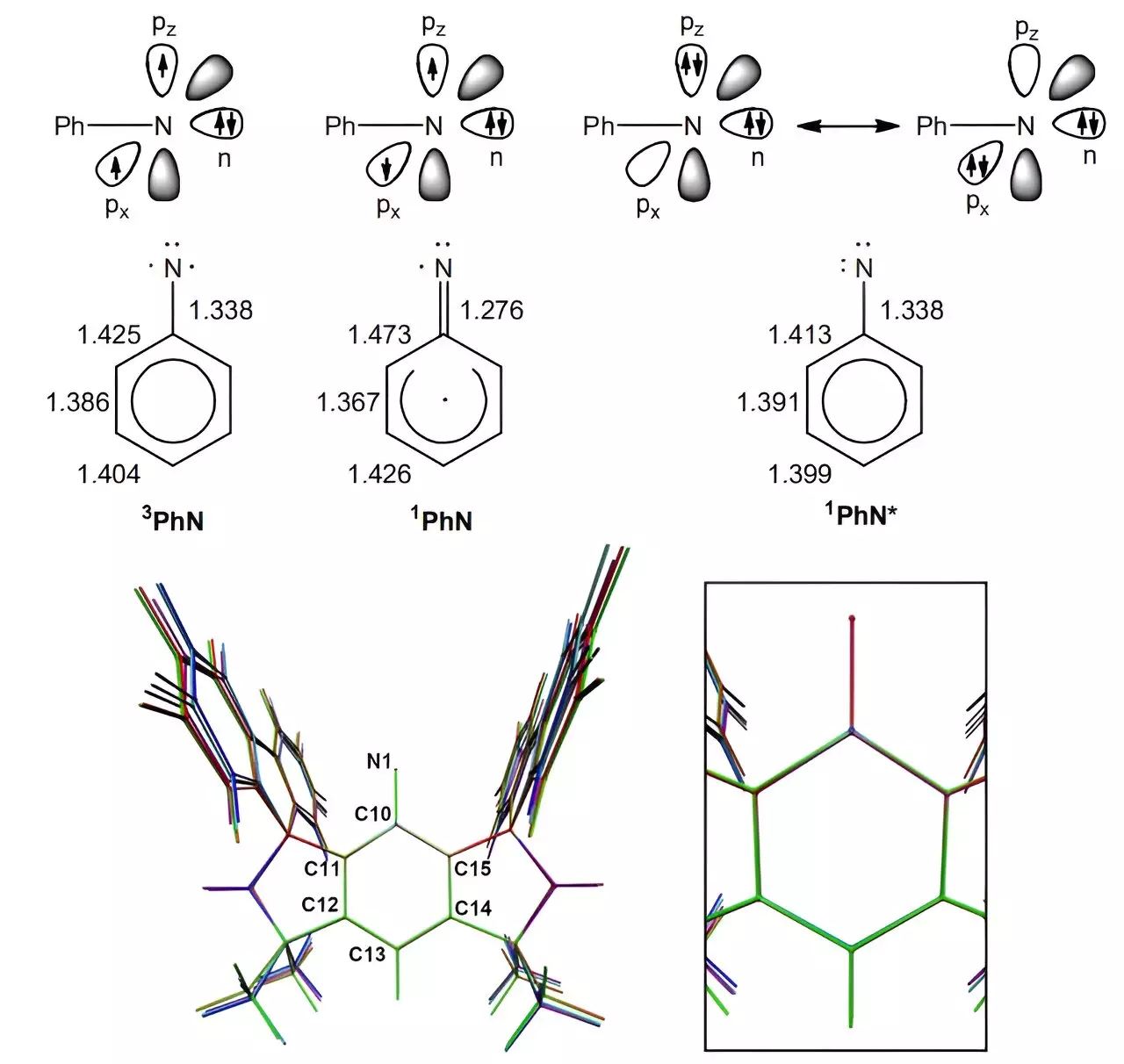
To achieve this groundbreaking result, the research team utilized a chemical scaffolding known as MSFluind. By employing this unique method, they were able to slow down the nitrene reactions significantly. The researchers separated the components in a reaction from accessing the nitrogen atom, allowing for a much slower process. This technique involved exposing a sample of MSFluindN3 to ultraviolet light, which reduced it to MSFluindN. Subsequent analysis using X-ray crystallography, electron paramagnetic resonance spectroscopy, and superconducting quantum interference device magnetometry confirmed the stability and longevity of the new nitrene.
The ability to study the characteristics of the slow-reacting nitrene for up to three days opens up new possibilities for exploring different applications in chemistry. The research team suggests that this newly developed technique could lead to the synthesis of new transition metal complexes. This breakthrough not only expands our understanding of nitrene chemistry but also provides a platform for further research and innovation in the field.
The development of a slow-reacting nitrene by the team of chemists at the University of Bremen represents a significant advancement in the field of chemistry. By overcoming the limitations of fast-reacting nitrenes, this new discovery has the potential to revolutionize the way we approach chemical reactions in various applications. The future of nitrene chemistry looks promising with the introduction of this groundbreaking research.

Leave a Reply