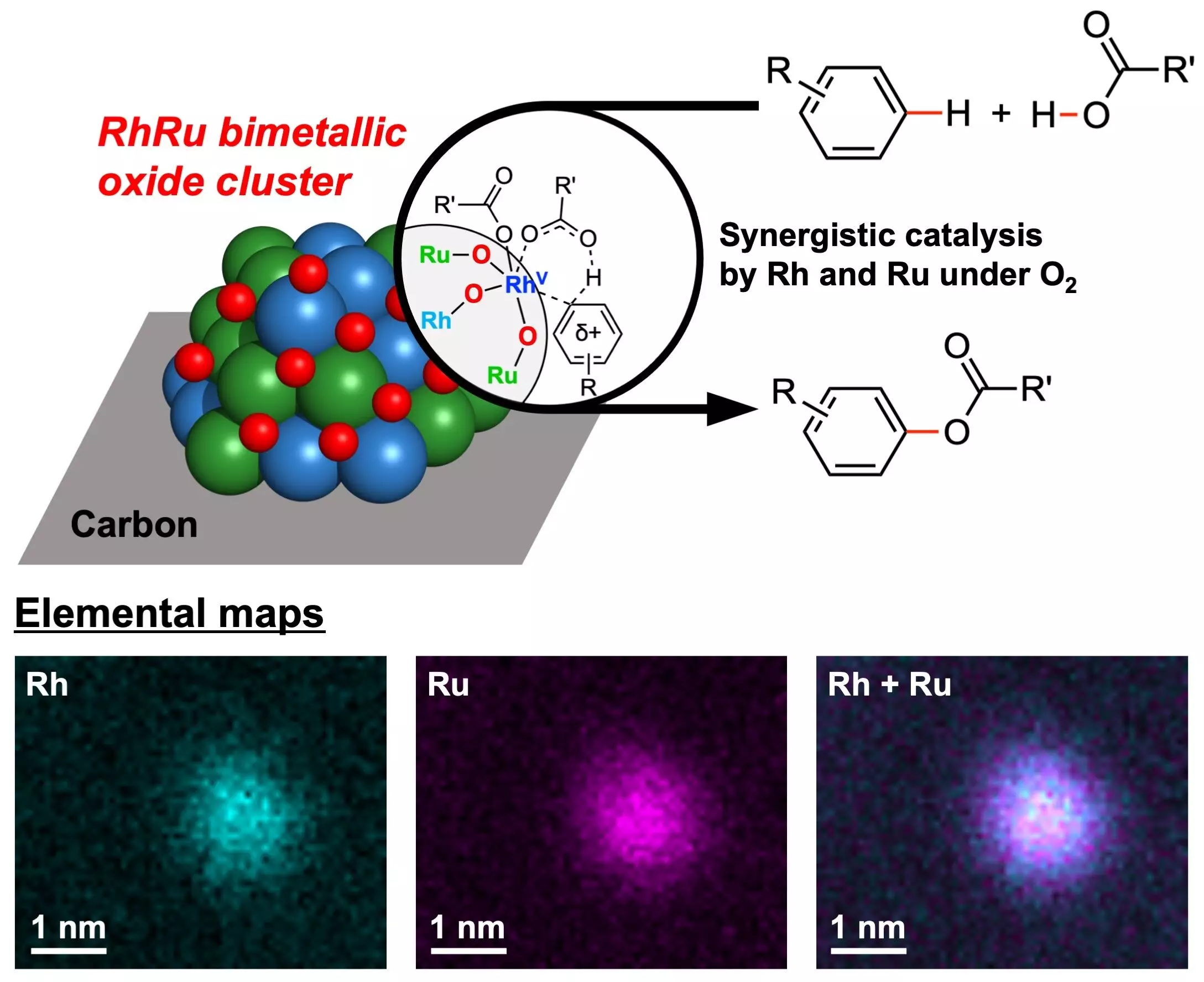
In a groundbreaking study published in the Journal of the American Chemical Society on June 6, chemists from Yokohama National University have unveiled a new catalyst that could revolutionize the field of green chemistry. This catalyst, known as RhRu bimetallic oxide clusters (RhRuOx/C), contains two noble metals – Rhodium (Rh) and Ruthenium (Ru) – and utilizes oxygen as the sole oxidant in ester-producing chemical reactions.
The Problem with Traditional Methods
Traditional methods of Cross-Dehydrogenative Coupling (CDC) reactions, which are essential in organic synthesis and industrial chemistry, often require hazardous oxidants such as hypervalent iodine reagents. These oxidants pose significant environmental and safety concerns, as they can be toxic to humans and animals, cause explosions, or produce harmful byproducts. As such, there has been a pressing need for a greener and more sustainable approach to these chemical reactions.
The RhRu bimetallic oxide clusters developed by the research team at Yokohama National University offer a promising solution to the problems associated with traditional CDC reactions. These catalysts, with a mean diameter of just 1.2 nm, have demonstrated remarkable efficiency in converting arenes and carboxylic acids into aryl esters using molecular oxygen as the sole oxidant. This not only eliminates the need for hazardous oxidants but also significantly reduces the environmental impact of the reactions.
The development of these new catalysts opens up possibilities for more sustainable and efficient chemical synthesis practices. The researchers plan to explore the use of these catalysts in other important chemical reactions, with the ultimate goal of establishing efficient and regioselective C–H functionalization reactions catalyzed by metal oxide clusters using molecular oxygen under mild conditions. This could lead to a new era of greener chemistry practices that are both environmentally friendly and economically viable.
The discovery of the RhRu bimetallic oxide clusters as efficient catalysts for CDC reactions represents a significant advancement in the field of green chemistry. By utilizing oxygen as the sole oxidant, these catalysts pave the way for more sustainable and environmentally friendly chemical reactions. With further research and development, these catalysts have the potential to revolutionize the way we approach chemical synthesis and contribute to a greener and more sustainable future for the industry.

Leave a Reply