In a groundbreaking study led by Prof. Zhang Guoqing from the University of Science and Technology of China (USTC) of the Chinese Academy of Sciences (CAS), researchers have unveiled a remarkable discovery of highly reactive photo-induced charge-transfer complexes (PCTCs) between amines and imides. This significant finding, published in the journal Chem, sheds light on the intricate processes involved in charge transfer between molecules.
Understanding Charge Transfer Complexes
Charge transfer between molecules is a crucial phenomenon in natural and synthetic systems, playing a vital role in processes such as photosynthesis, respiration, organic synthesis, and energy conversion applications. Despite extensive research in this field, creating stable and light-responsive charge-transfer complexes in artificial systems has posed a significant challenge.
The Discovery of PCTCs
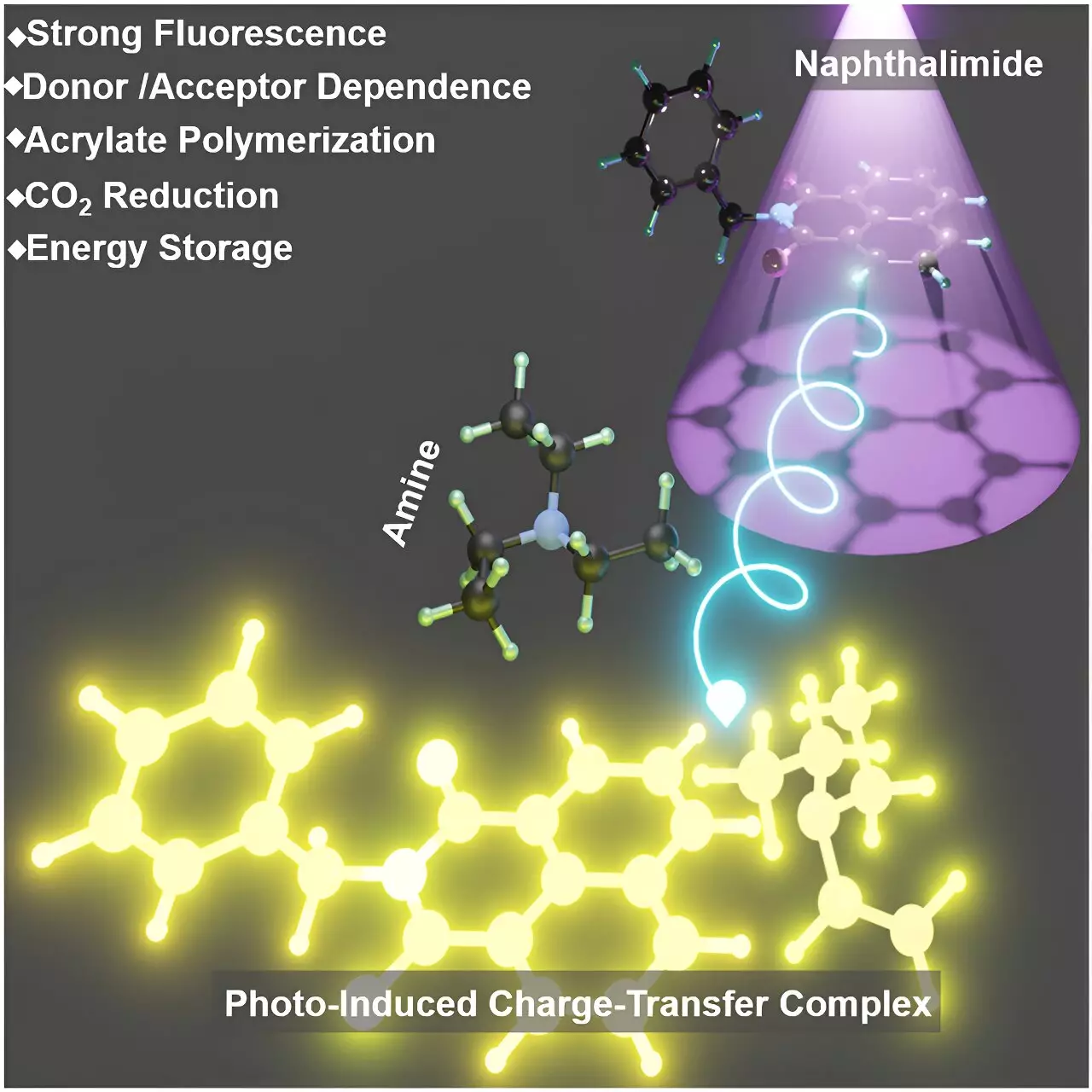
The research team discovered that aromatic imides and alkyl amines, which had minimal interaction in their ground state, formed stable PCTCs when exposed to UV light. This interaction led to the formation of a highly fluorescent complex resembling a Meisenheimer complex, offering valuable insights into complex photochemical processes.
Utilizing various spectroscopic techniques, such as high-resolution mass spectrometry and time-resolved spectroscopy, the researchers confirmed the formation and stability of these PCTCs. Through a series of experiments, they explored the mechanism of PCTC formation and observed distinct absorption bands and enhanced fluorescence upon UV irradiation, indicating the successful formation of these complexes.
The application of PCTCs extended beyond theoretical research, demonstrating practical utility in polymerization reactions under UV light. The researchers showcased the potential of PCTCs in creating new polymeric materials with unique properties. Moreover, the complex exhibited efficacy in reducing carbon dioxide, a critical reaction for environmental sustainability and developing renewable energy sources.
One of the most intriguing aspects of PCTCs was their ability to store UV energy and release it in the absence of light. This unique capability allowed processes that traditionally relied on continuous light exposure to proceed even in the dark, opening up new avenues for applications in polymer science, environmental technology, and energy storage.
The discovery of highly reactive PCTCs between amines and imides under UV light represents a significant advancement in understanding photo-induced charge-transfer processes. This breakthrough not only contributes to fundamental scientific knowledge but also holds promising potential for practical applications in various fields, paving the way for innovative solutions to pressing global challenges.

Leave a Reply