In a groundbreaking study conducted by a team of researchers from Pohang University of Science and Technology (POSTECH), the Korea Research Institute of Chemical Technology, and Chonnam National University, a new technique for the separation of well-mixed mixtures has been developed. Led by Professor Jee-hoon Han from the Department of Chemical Engineering at POSTECH, the team created a technology for the efficient synthesis and purification of ionic liquids, with their research being featured as the cover paper in the online edition of Industrial & Engineering Chemistry Research.
Ionic liquids are salts that remain in a liquid state at room temperature or even at relatively low temperatures due to strong electrical interactions between their ions. These unique properties, such as nonflammability, low volatility, and thermal and chemical stability, make them valuable for various industrial applications including catalysts and electrolytes. One of the most well-known ionic liquids is [bmim][BF4], known for its high stability and low toxicity. However, the complex and expensive process of removing impurities such as lithium chloride (LiCl) during synthesis has hindered the commercialization of this technology.
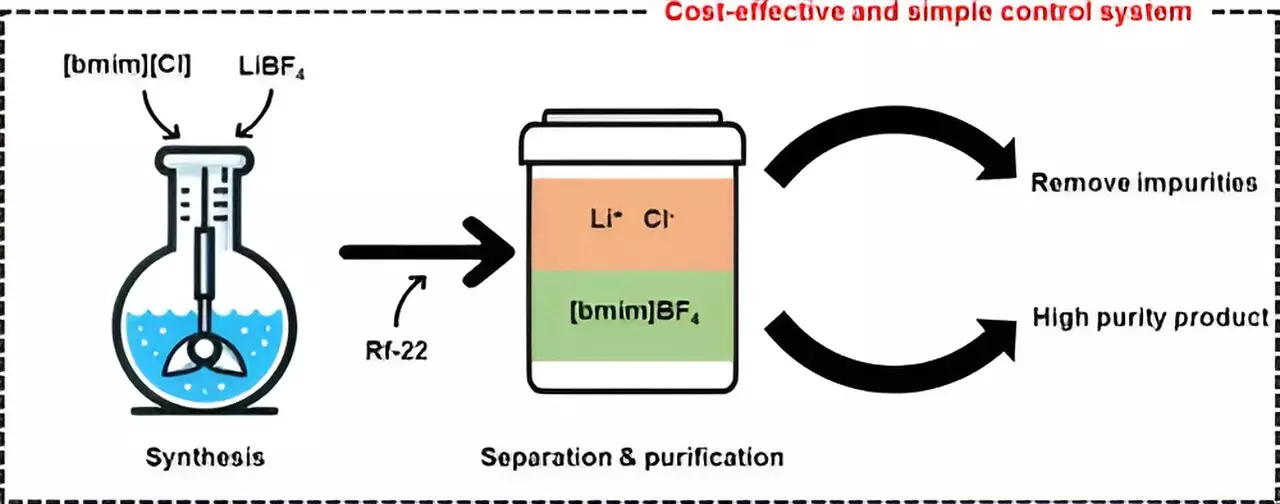
In their study, the researchers utilized halocarbon refrigerants, specifically chlorodifluoromethane (Rf-22), to synthesize the ionic liquid [bmim][BF4] more economically and efficiently than traditional methods. By using Rf-22 as a phase separation mediator, they were able to induce a mixture containing methylimidazole to separate into two distinct layers, similar to the separation of oil and water. Through the manipulation of the ratios of [bmim][BF4], water, and halocarbon mixtures, the team observed phase separation and applied the collected data to a ternary phase diagram model.
Using the ternary phase diagram modeling, the researchers were successful in producing high-purity [bmim][BF4] with a purity exceeding 99%. Furthermore, they were able to recover and recycle the layer containing methylimidazole, which did not participate in the synthesis reaction. To evaluate the economic feasibility of the purification technology developed in this study, the team conducted process simulations and determined that the minimum selling price for producing 1 ton of [bmim][BF4] per day would be about $12,000 per ton. This cost analysis highlighted the competitiveness of the developed technology compared to existing process technologies, showcasing its potential for commercialization.
The development of this innovative technique for the synthesis and purification of ionic liquids marks a significant advancement in the field of chemical engineering. The utilization of halocarbon refrigerants and ternary phase diagram modeling has enabled the production of high-purity [bmim][BF4] in a more efficient and cost-effective manner. The successful recovery and recycling of components further enhance the sustainability of the process. With promising results in both purity levels and economic feasibility, this technology holds great potential for widespread commercial use in various industrial applications.

Leave a Reply