Rare-earth metals play a crucial role in our technology-driven world, featuring prominently in applications ranging from clean energy systems to advanced healthcare technologies. Despite their name, these metals—including the 15 rare lanthanides—are comparatively abundant in the Earth’s crust, akin to elements like copper and lead. The challenge extends beyond their abundance; what makes these metals “rare” is the complexity and resource intensity of extracting them from their ores. Recent studies from the Oak Ridge National Laboratory (ORNL), in collaboration with Vanderbilt University, have unveiled a significant advancement in the purification process—introducing a unique chemical “chameleon” that could reshape how lanthanides are isolated.
Lanthanides possess similar physical and chemical characteristics, which complicates their separation during the purification process. The success of applications utilizing these metals hinges on the ability to isolate them from mixtures, a feat requiring advanced techniques in separation chemistry. The traditional method relies on the use of ligands—specific molecules designed to selectively bind to individual lanthanide ions. Unfortunately, this process is typically inefficient, not only requiring multiple separation stages but also generating considerable waste. As Subhamay Pramanik, a radiochemist at ORNL, articulates, the minute differences in size and charge among lanthanides pose substantial hurdles in efficient extraction, making the search for better separation techniques imperative.
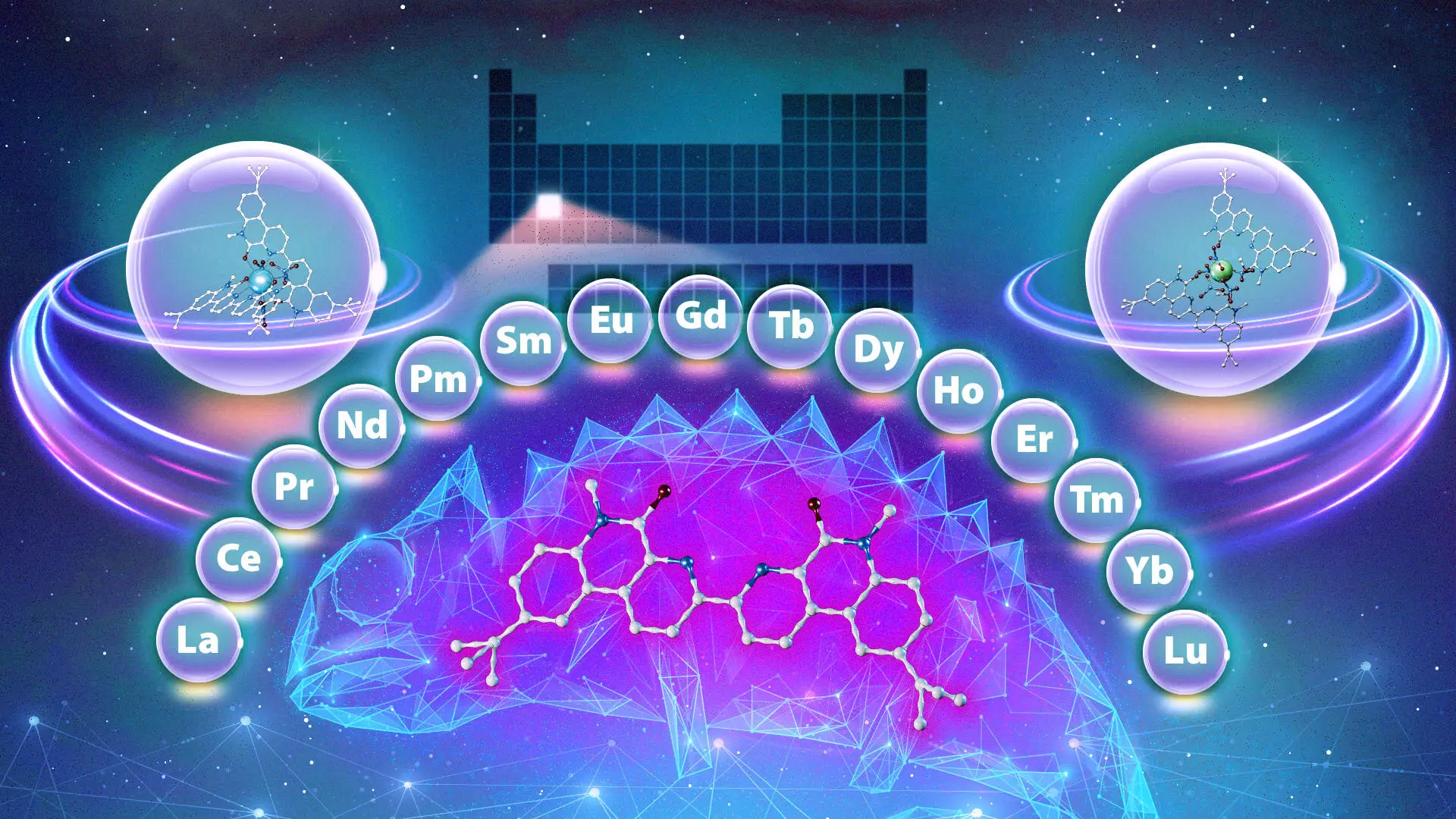
In a remarkable twist, researchers at ORNL have discovered a new ligand with “chameleon-like” properties; it adapts its binding behavior based on environmental conditions. While conventional ligands display a consistent preference for either light or heavy lanthanides, this novel compound offers unprecedented versatility. Depending on the acid concentration in the solution and the duration of interaction, the ligand can selectively bind different lanthanides, drastically reducing the number of steps required for effective separation.
The implications of this discovery are profound. By lowering the number of necessary separation processes, not only could operational costs decrease, but the environmental impact of the purification process could also alleviate. The “chameleon” ligand, by its design, effectively challenges the established norms of separation chemistry.
Understanding the mechanisms through which the chameleon ligand operates is crucial for future research and applications. The adaptability of the ligand is notable because it allows for flexibility in extracting lanthanides in any order, regardless of their typical density variations. This suggests that researchers may be able to employ a single ligand for different separation tasks, which tilts the conventional paradigm of using varied ligands for different lanthanides. As Santa Jansone-Popova from ORNL explains, this adaptability is both exciting and unique, opening avenues for more efficient purification strategies that could transform industrial practices.
The revelation of the chameleon’s capabilities marks just the beginning of exploration into lanthanide-binding compounds. With established knowledge about this ligand, future research may focus on synthesizing new compounds that can further streamline separation processes. The study demonstrates that even structurally similar compounds can exhibit vastly different behaviors, urging chemists to explore the potential diversity of ligands available for rare-earth metal applications.
As ORNL researcher Ilja Popovs states, the findings bolster our understanding of ligand behavior, thereby fostering innovative solutions to longstanding problems in the field of separation science. Researchers might now harness the principles of this unique chameleon-like behavior to design ligands optimized not only for rare-earth metals but also for a range of applications beyond current limitations.
The advancements heralded by ORNL’s discovery of the chemical chameleon signify a pivotal moment in the field of lanthanide purification. By optimizing rare-earth metal separation processes, this research stands to benefit numerous technological sectors, enhancing the sustainability and efficiency of metal extraction. As global demand for clean energy solutions and advanced technologies continues to rise, the refinement of these chemicals could play an essential role in addressing both economic and environmental challenges. The journey of this chameleon ligand is only beginning, promising a bright future for materials science and rare-earth metal applications, while pushing the boundaries of our understanding in the field.

Leave a Reply