Esters, the compounds responsible for the sweet smell of fruits like strawberries, are widely used in various industries. However, the process of breaking down esters to produce alcohols and other chemicals can be costly and harmful to the environment. Traditional methods of ester reduction involve the use of highly reactive and difficult to handle metal reductants.
Researchers from the National Institutes of Natural Sciences (NINS) in Japan have developed a novel approach to ester reduction using light as an energy source. By utilizing sustainable photocatalysts, the researchers aim to promote a more environmentally friendly and cost-effective method of reducing esters. Photocatalysts are known for their ability to activate during light excitation, facilitating an electron transfer process without the need for reactive metal reductants.
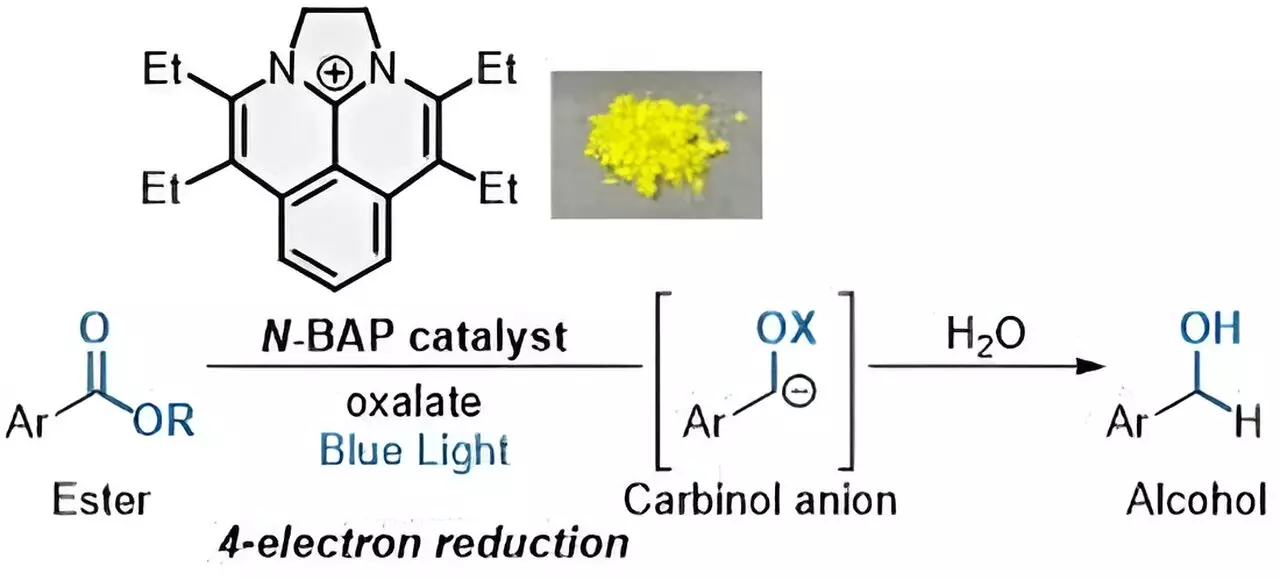
In their study published in the Journal of the American Chemical Society, the researchers introduced a new photocatalyst called “N-BAP.” This catalyst, when exposed to blue light, initiates a reaction that leads to the reduction of esters to form alcohols. By adding oxalate as a traceless reductant, the N-BAP catalyst enables a rapid sequential four-electron reduction process, resulting in the desired alcohols. This innovative approach overcomes the limitations of traditional single-electron transfer (SET) processes and opens up new possibilities for ester reduction.
The use of photocatalysts in ester reduction aligns with the United Nations’ Sustainable Development Goals (SDGs) by promoting environmentally friendly methods in organic synthesis. By utilizing visible light as an energy source and avoiding the use of expensive and non-renewable noble metals, the researchers have set a new standard for sustainable chemical reactions. The successful reduction of esters to alcohols through a quadruple SET process represents a significant advancement in the field of photocatalytic reactions.
Moving forward, researchers aim to further explore the potential applications of the N-BAP catalyst in other organic synthesis reactions. The ability to achieve a rapid and sequential four-electron reduction of esters opens up new possibilities for the development of novel chemicals and pharmaceutical products. By harnessing the power of light as an energy source, scientists are paving the way for a more sustainable and efficient approach to chemical reactions.
The breakthrough in ester reduction using light as an energy source represents a significant advancement in the field of organic synthesis. The development of the N-BAP catalyst and the successful quadruple SET process demonstrate the potential for sustainable and environmentally friendly chemical reactions. With further research and innovation, this novel approach to ester reduction has the potential to revolutionize the way we produce chemicals and pharmaceuticals in the future.

Leave a Reply