For many years, chemists have been familiar with Michael addition reactions, but the concept of anti-Michael addition reactions has always been elusive. The difficulties lie in the higher electrophilicity of the β-position compared to the α-position in α,β-unsaturated carbonyl compounds. Previous attempts to overcome these challenges through intramolecular reactions and the introduction of strong-electron withdrawing groups at the β-position have fallen short in providing an ideal solution for synthesizing complex molecules using the anti-Michael reaction.
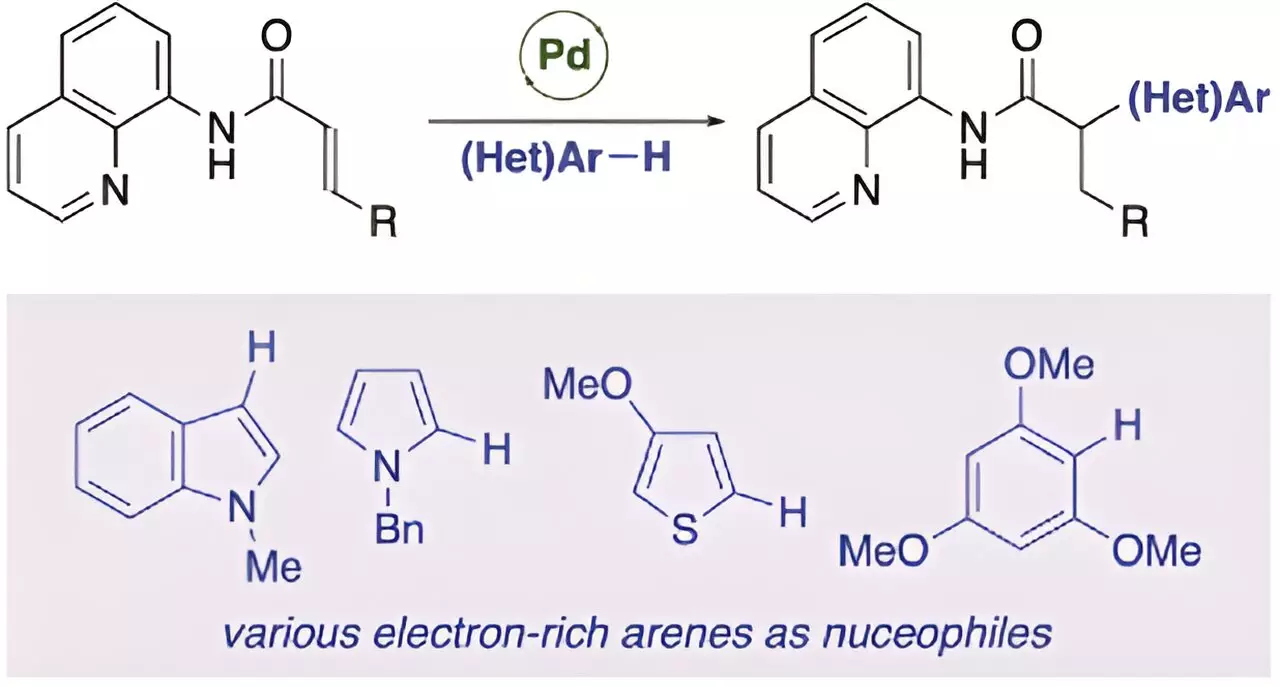
However, a recent study led by Professor Takanori Matsuda from the Department of Applied Chemistry at Tokyo University of Science, Japan, along with Ryota Moro and Assistant Professor Hirotsugu Suzuki from the University of Fukui, Japan, has achieved a significant milestone in the field. Their groundbreaking research focused on the palladium-catalyzed anti-Michael addition reaction of acrylamides, marking the first example of an anti-Michael-type addition reaction.
The researchers discovered that the presence of a catalytic amount of palladium(II) trifluoroacetate (Pd(TFA)2) could facilitate the anti-Michael addition of indole to acrylamide with an aminoquinoline group as a directing group, resulting in the high-yield production of the addition product. By introducing a directing group into the α,β-unsaturated carbonyl compound, the researchers were able to stabilize the reaction intermediate and promote the anti-Michael type addition reaction.
The implications of this research are far-reaching, particularly in the field of organic synthesis. The anti-Michael type addition reaction has the potential to streamline the synthesis of α-substituted carbonyl compounds, which are commonly used in pharmaceuticals. With 100% atomic efficiency and the ability to produce a wide range of compounds, this new method holds promise for the pharmaceutical industry and beyond.
The discovery of the palladium-catalyzed anti-Michael addition reaction represents a significant advancement in the field of organic synthesis. By overcoming the challenges associated with anti-Michael reactions, this research opens up new possibilities for the efficient and sustainable synthesis of complex organic compounds, with potential applications in drug discovery, materials science, and beyond. The impact of this breakthrough is likely to be felt across various industries, paving the way for innovative and more efficient chemical transformations.

Leave a Reply