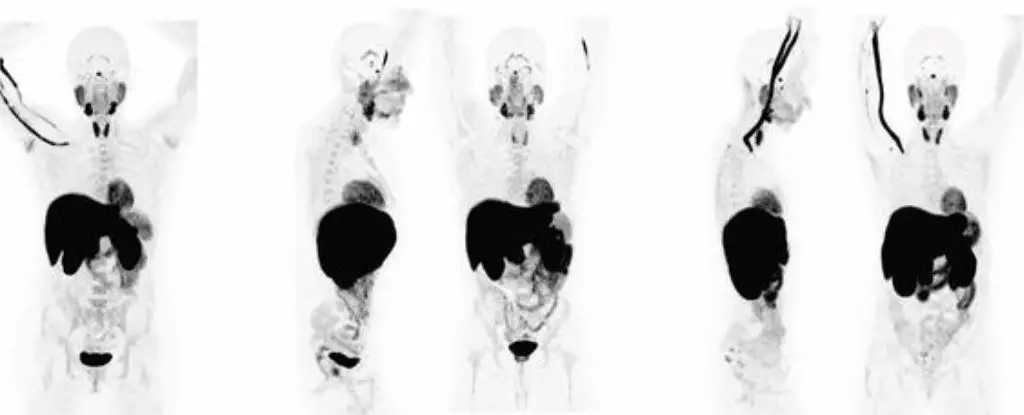
Long COVID, a condition characterized by lingering symptoms following a SARS-CoV-2 infection, has long been a mysterious and debilitating illness. Contrary to early beliefs that it may be psychosomatic in nature, a recent study has shed light on the biological underpinnings of this complex disease. Researchers from the University of California, San Francisco, CellSight Technologies, and Kaiser Permanente South San Francisco Medical Center conducted PET imaging tests on 24 patients who had recovered from COVID-19. The results were striking, revealing abnormal T cell activity in various parts of the body, including the brain stem, spinal cord, bone marrow, and more.
The study found that the abnormal T cell activity was present not only in patients experiencing long COVID symptoms but also in those who had fully recovered from the acute phase of the virus. This suggests that even a seemingly mild COVID-19 infection can have long-lasting effects on the immune system. The persistent changes in T cell activity observed in multiple organs, even years after the initial infection, raise concerns about the potential long-term consequences of COVID-19 on immune homeostasis. Additionally, the increased uptake of the PET tracer in the lungs and pulmonary artery walls of patients with ongoing respiratory issues highlights the systemic impact of the virus on various tissues.
While the findings are correlational, they provide compelling evidence that long COVID is linked to the persistence of the SARS-CoV-2 virus in the body and abnormal immune activity. The presence of the virus in tissues throughout the body, as seen in COVID autopsies, underscores the potential for long-term viral reservoirs and immune dysregulation in patients with long COVID. Moreover, the discovery of T cell abnormalities in the spinal cord and brain stem suggests a neurological component to the illness, challenging previous notions of COVID-19 as a transient acute infection.
One of the biggest challenges in addressing long COVID lies in its diagnosis, given the wide range of symptoms that can manifest in patients. With over 200 reported symptoms, many of which overlap with other conditions, it is crucial to develop specific diagnostic criteria to identify and treat individuals with long COVID. The presence of biomarkers of inflammation and immune activation in patient blood samples post-infection further complicates the diagnostic process. Additionally, the overlap between long COVID and conditions like chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) raises questions about the relationship between these illnesses and the potential for shared treatments.
As our understanding of long COVID continues to evolve, it is clear that a multidisciplinary approach is needed to unravel the complexities of this illness. By exploring the biological mechanisms at play, such as abnormal T cell activity and viral persistence, we can pave the way for targeted therapies and interventions. The recent advancements in immune mapping techniques offer hope for further insights into the immune effects of long COVID on the body. Moving forward, larger cohort studies will be essential to validate these findings and explore potential treatment strategies for individuals living with the long-lasting effects of COVID-19.

Leave a Reply