The University of Liverpool researchers have made significant progress in the field of converting carbon dioxide (CO2) into valuable fuels and chemicals. This breakthrough marks a crucial step towards achieving a sustainable net-zero economy by utilizing a plasma-catalytic process for CO2 hydrogenation to methanol at room temperature and atmospheric pressure.
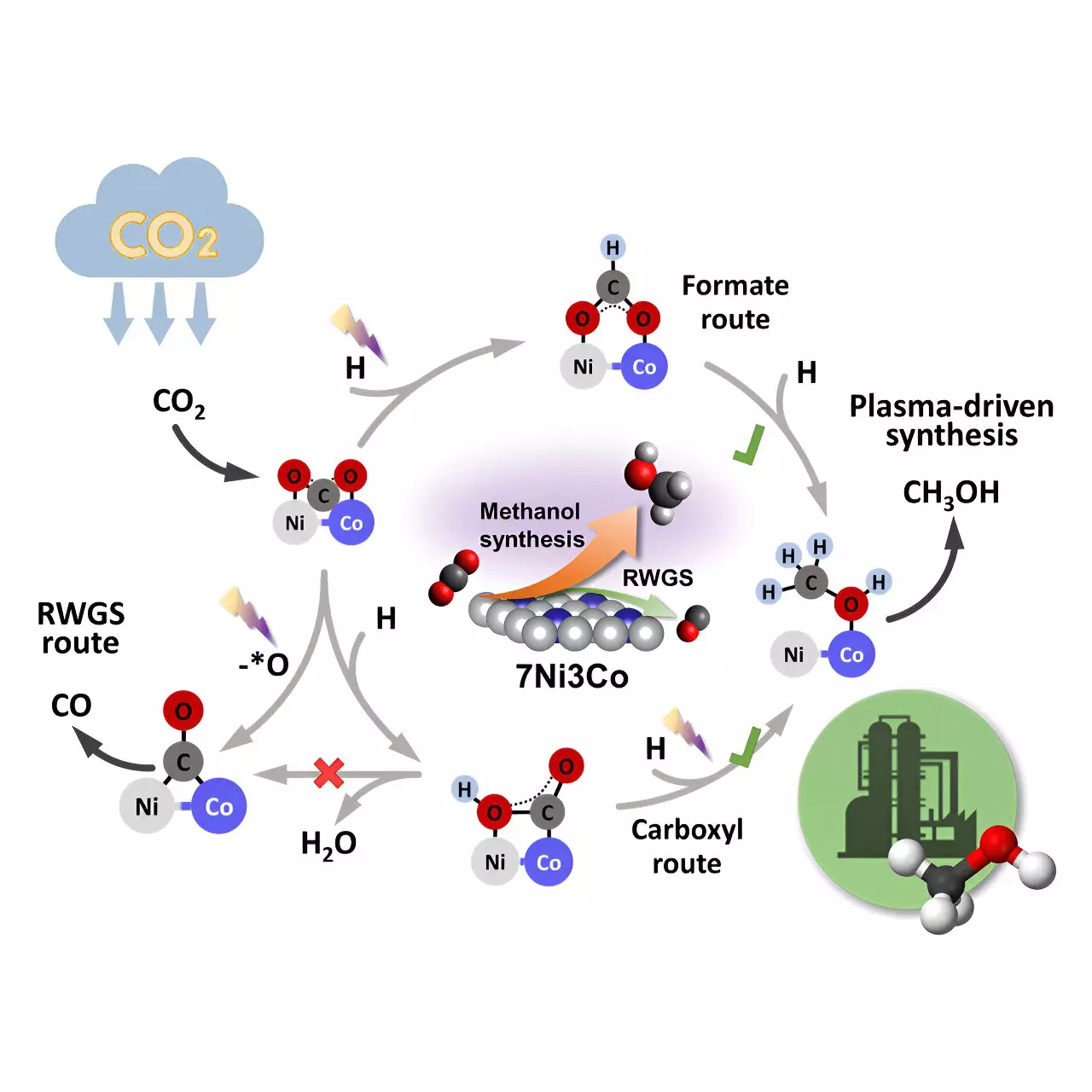
Unlike traditional thermal catalysis, which requires high temperatures and pressures, the novel plasma-catalytic process achieves an impressive single-pass 46% selectivity for methanol and 24% CO2 conversion at a mere 35 °C and 0.1 MPa. By using a bimetallic Ni-Co catalyst within a non-thermal plasma reactor, strong chemical bonds of inert molecules like CO2 can be activated, enabling chemical reactions under mild conditions.
Plasma catalysis offers a flexible and decentralized solution for CO2 hydrogenation to methanol under ambient conditions. The ability to utilize intermittent renewable electricity for decentralized production of fuels and chemicals showcases the potential of plasma-based modular systems. This process not only reduces capital costs compared to traditional thermal catalytic methods but also provides a viable route for utilizing renewable energy sources in the production of synthetic fuels.
In situ plasma-coupled Fourier transform infrared (FTIR) characterization and density functional theory (DFT) calculations revealed that the bimetallic Ni-Co interface is the primary active center for methanol synthesis. The Eley-Rideal (E-R) mechanism is responsible for CO2 adsorption and hydrogenation to produce a variety of intermediates. The formate and carboxyl routes are critical for methanol formation, while the reverse water-gas shift (RWGS) and CO hydrogenation pathways are less favorable on the Ni-Co sites.
The Promise of Bimetallic Catalysts
The precise control of Ni-Co sites in bimetallic catalysts shows promise for tailoring the weight of each reaction pathway. By promoting asymmetric adsorption of CO2 molecules at the bimetallic interfaces, the distribution of products can be effectively modulated. This research highlights the significant potential of plasma catalysis as an emerging electrification technology for sustainable CO2 conversion and fuel production.
The University of Liverpool research team’s pioneering work opens up promising avenues for future research and industrial applications in the field of catalytic CO2 conversion. With the ability to perform these reactions at ambient conditions using a modular and scalable plasma system, the chemical industry has an attractive alternative for decentralized fuel and chemical production. The use of plasma-based systems powered by intermittent renewable electricity further enhances the feasibility of sustainable fuel and chemical production.
The University of Liverpool researchers have made substantial advancements in plasma catalysis for CO2 conversion, offering a sustainable and innovative solution for the production of valuable fuels and chemicals. This pioneering work is a major step forward in addressing the challenge of a sustainable future and provides promising opportunities for further research and industrial applications.

Leave a Reply