In today’s ever-evolving world, plastics are ubiquitous, and among them, polypropylene stands out as one of the most widely utilized forms. Found in everything from food containers to critical medical devices, the demand for this essential material is soaring. This surge in popularity has inevitably led to an increased demand for propylene, the key chemical precursor required for the manufacturing of polypropylene. What many may not realize is that propylene can be derived from propane, a familiar natural gas often associated with backyard barbeques. However, the challenge has always been the efficiency and eco-friendliness of the manufacturing process.
Transforming the Catalytic Landscape
Recent research conducted by the U.S. Department of Energy’s Argonne National Laboratory and Ames National Laboratory has introduced a groundbreaking method to synthesize propylene, optimizing both energy use and reaction speed. Their findings, published in a prestigious scientific journal, present a significant departure from traditional catalysis, challenging conventional wisdom and techniques that have dominated the field for years.
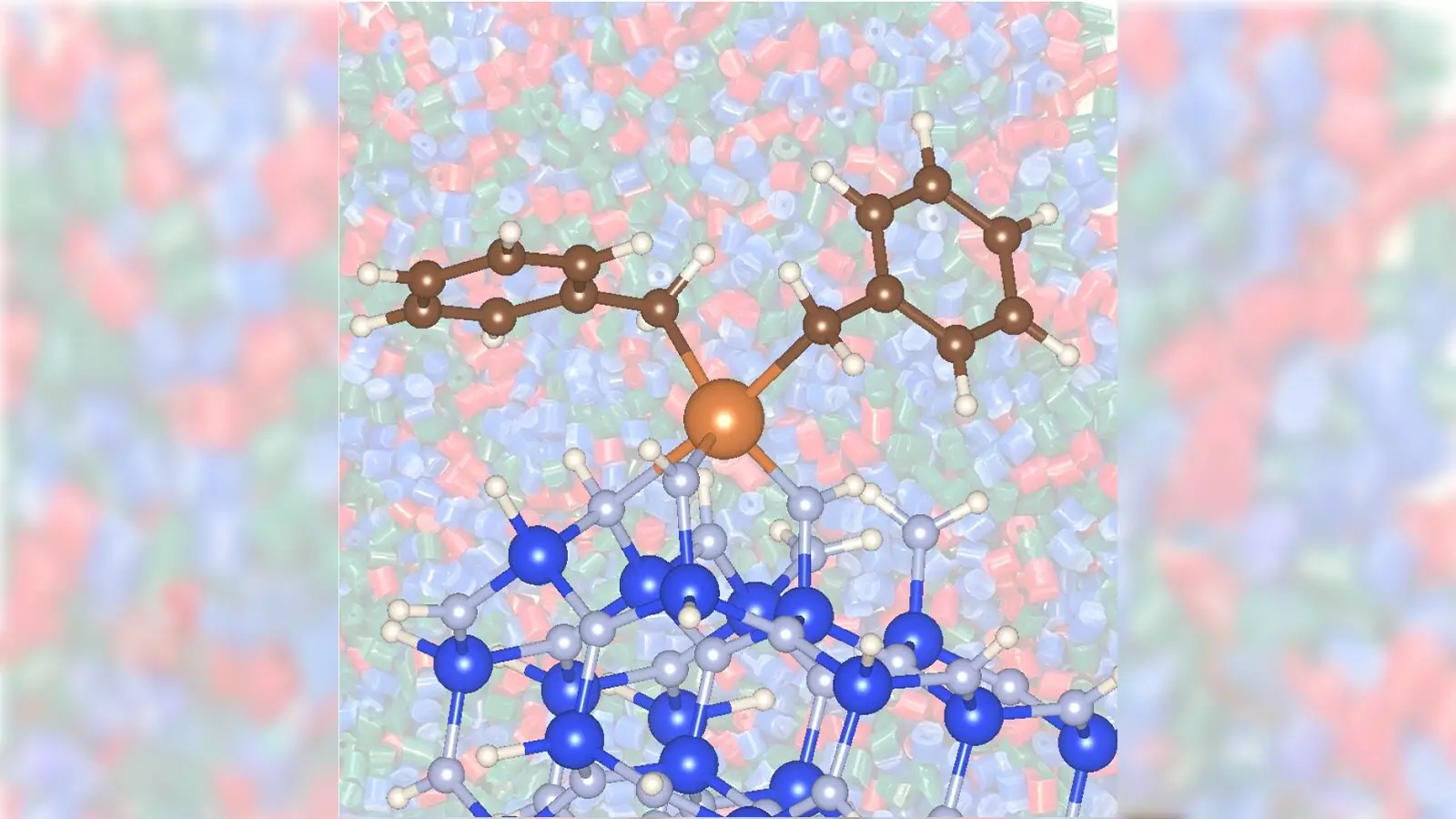
Traditionally, the catalytic conversion of propane to propylene has relied heavily on precious metals such as platinum or the use of metal catalysts like chromium, which require high temperatures and consume considerable energy. However, the collaborative team at Argonne and Ames has uncovered that a combination of zirconium with silicon nitride offers a more effective and sustainable approach, significantly reducing both energy expenditures and toxic byproducts.
Insights into Catalysis: The Role of Nontraditional Materials
Lead researchers David Kaphan and Max Delferro pioneered this novel study, revealing the vital role that nontraditional materials, like silicon nitride, can play in enhancing catalytic efficiency. Their research not only highlights the capabilities of zirconium as a catalyst but also emphasizes the significant impact that the choice of support material can have. Unlike traditional catalyst supports, which are often limited to oxides, silicon nitride has shown a remarkable capability to facilitate faster reaction rates, proving that innovative materials can substantially transform catalysis.
In their experiments, the researchers achieved successful catalytic conversion at remarkably lower temperatures—842 degrees Fahrenheit compared to the conventional 1,022 degrees. This reduction is not merely anecdotal but has far-reaching implications for energy consumption and environmental impact, particularly when one considers the formidable challenge that greenhouse gas emissions pose.
Pioneering the Future of Catalytic Processes
One of the standout findings from this research is its potential as a template for future catalytic processes beyond the conversion of propane to propylene. Kaphan expressed optimism regarding the implications of this work, suggesting that the enhanced reactivity enabled by low-cost metals on nitride surfaces could revolutionize the chemical industry. By harnessing this approach, we could witness a significant shift in how we manufacture not only plastics but also a vast array of chemical products.
Moreover, the collaboration between Argonne and Ames for this project illustrates a unique confluence of expertise, combining advanced material characterization techniques with innovative experimental research. Utilizing advanced facilities such as Argonne’s Advanced Photon Source, the researchers were able to obtain crucial insights into the interaction between zirconium and silicon nitride, further elucidating the mechanisms behind their catalytic success.
The Value of Collaborative Research and Future Prospects
Research often thrives on collaboration, as this project illustrates. With an array of scientists contributing their specialized knowledge, the success of this research underscores the importance of teamwork in addressing complex scientific challenges. The study is a testament to the power of collective expertise in propelling forward groundbreaking innovations in catalysis.
As industries begin to embrace this new methodology for producing propylene, we can anticipate positive shifts across several sectors, especially in plastics and material fabrication. This transition not only promises to make processes more efficient but also stands to minimize the environmental impact associated with traditional methods.
The innovative strides made in catalysis by Argonne and Ames represent a significant leap toward more sustainable industrial practices. With the dual goals of reducing costs and environmental footprints, the future of propylene production looks not just promising, it looks revolutionary. As we move forward, this research provides a vital framework for tackling not only the challenges of today but also those of tomorrow in the ever-critical fight against climate change.

Leave a Reply