In an era increasingly defined by environmental awareness and sustainability, researchers at the Ulsan National Institute of Science and Technology (UNIST) have made a significant breakthrough that could transform the way we combat pollution. Under the guidance of Professor Jaeheung Cho, the team has succeeded in engineering a novel catalyst that not only emulates the natural processes of metalloenzymes but does so with unprecedented efficiency. This research, published in the Journal of the American Chemical Society, represents a pivotal step toward cleaner chemical processes and offers a promising solution to longstanding environmental issues caused by harmful hydrocarbons.
Lessons from Nature: Designing a Bio-Inspired Catalyst
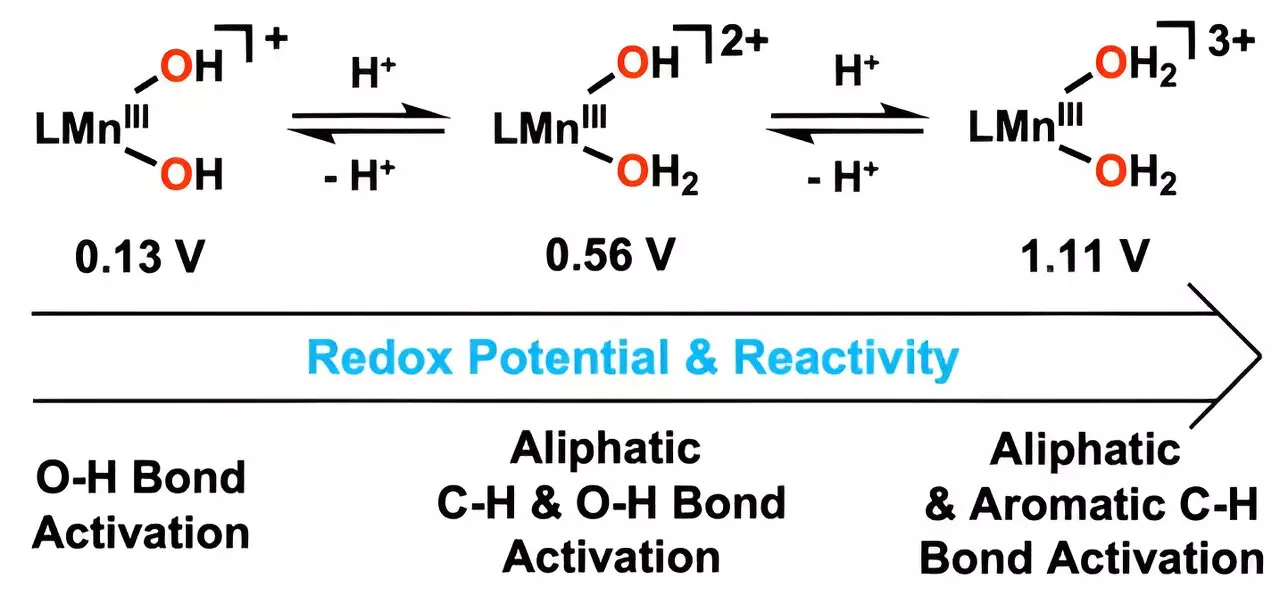
The catalyst in question borrows its design principles from the intricate mechanisms of natural enzymes, particularly their ability to oxidize hydrocarbons—substances notorious for their toxic effects on both human health and ecosystems. By integrating water molecules into a manganese-based catalyst, the research team has created a means of breaking down carbon-hydrogen (C-H) bonds at considerably lower temperatures than traditional methods. This innovation not only enhances reaction efficiency but also aligns perfectly with the global push for energy conservation and reduced greenhouse gas emissions. The ability to perform such complex reactions at lower temperatures signifies a potential reduction in energy consumption in industrial applications.
A Groundbreaking Methodology
Central to the effectiveness of this new catalyst is the strategic modification of the hydroxo ligand. By incorporating hydrogen ions, the research has managed to boost manganese’s reduction potential, a key factor in accelerating the oxidation process. This enhancement allows for the effective breakdown of resilient carbon-hydrogen bonds situated within compounds like anthracene—an elemental component often linked with environmental pollution. Moreover, the catalyst’s ability to decompose difficult-to-treat aromatic hydrocarbons suggests its versatility across various applications, enhancing its appeal for industrial use.
Potential for Industrial Transformation
Professor Cho has articulated a vision that extends beyond academic achievement; he foresees this research spearheading a new era for industrial catalysis. The ability to control manganese’s reduction potential implies that we could develop a suite of catalysts tailored to tackle diverse hydrocarbon challenges. This research not only stands as a testimony to human ingenuity and the relentless pursuit of solutions to pressing environmental issues but also aligns with contemporary ideals of sustainability and efficiency.
As industries grapple with regulatory pressures and shifts in public sentiment toward sustainability, innovations such as this catalyst position themselves at the forefront of transformative change. Their capacity to mitigate pollution while reducing energy demands might very well be the linchpin needed to navigate the complexities of modern production processes.
The team’s accomplishments at UNIST highlight the potential of scientific inquiry to yield practical innovations that address environmental crises. As the world continues to seek sustainable solutions, this catalyst serves as not just a scientific breakthrough but a beacon of hope for a greener future.

Leave a Reply