The pursuit of efficient, stable, and economically viable catalysts for energy conversion processes has driven significant research in the field of electrocatalysis, particularly in oxygen evolution reactions (OER). OER is crucial in various applications, such as water splitting and fuel cells, where it facilitates the generation of oxygen from water molecules. A novel approach has recently been introduced by a team of researchers, who unveiled an innovative electrocatalyst leveraging the rare earth element erbium (Er). This development not only promises enhanced performance but also positions itself as a competitive alternative to precious metal-based catalysts.
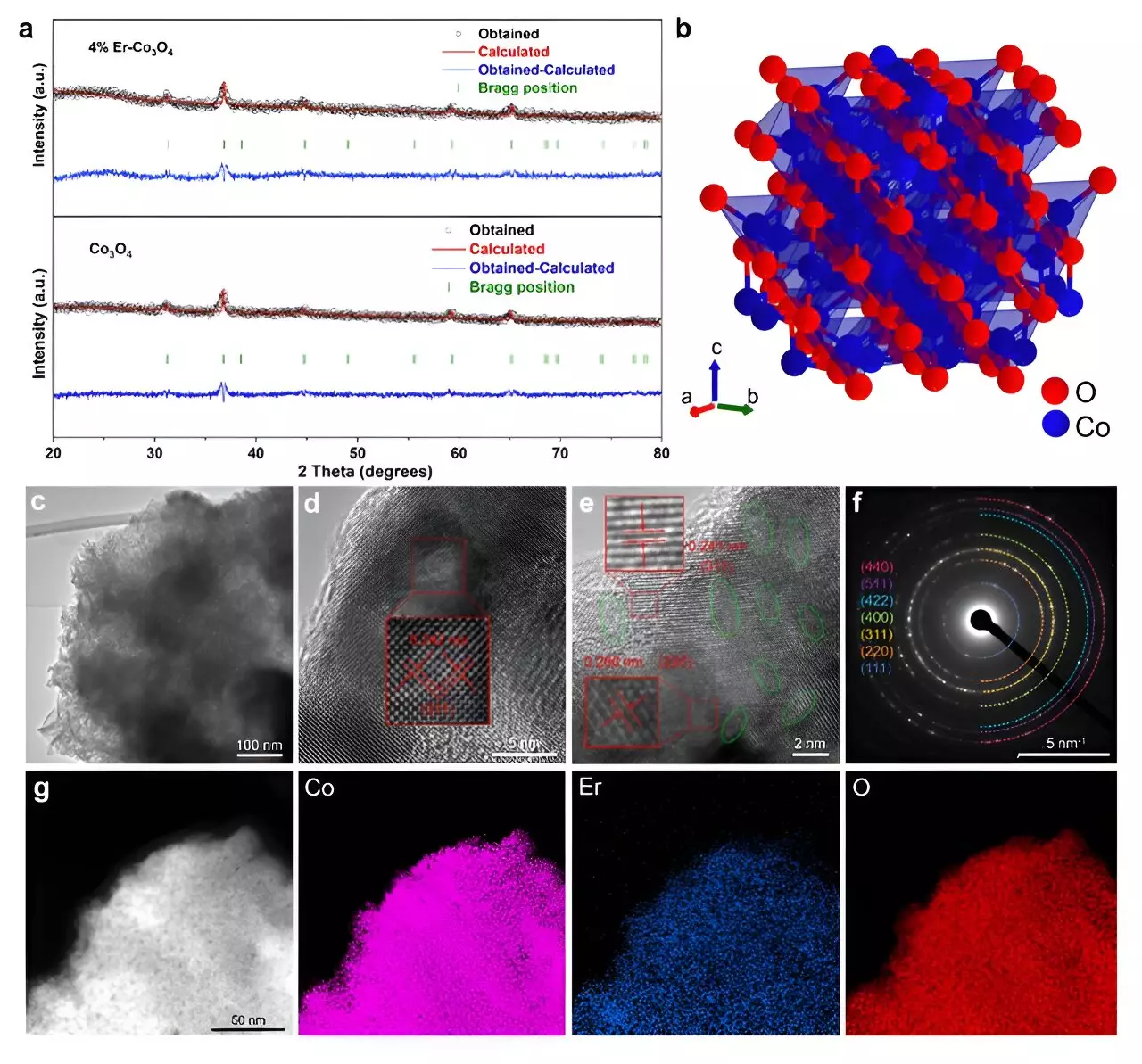
The research focuses on enhancing the traditional cobalt oxide catalyst, specifically Co3O4, by doping it with erbium. This strategic integration of Er into the cobalt oxide framework was documented in the prestigious journal ACS Catalysis and celebrated as an Editors’ Choice selection, signifying its relevance and potential impact on the scientific community. The conventional use of cobalt as a catalyst has often been hindered by its moderate efficiency in acidic conditions, stemming from its inherent crystal structure. However, by introducing Er into the mix, the research team led by Tianyi Wang demonstrated that catalytic performance could be significantly boosted.
The results of this work are compelling. The erbium-doped Co3O4 catalyst exhibited an impressive overpotential of just 321 mV at 10 mA cm², coupled with extraordinary stability over prolonged periods, maintaining performance consistently for over 250 hours. This durability and efficiency were found to be on par with, and in some instances superior to, established iridium-based catalysts, which are usually regarded as the standard for performance but come with steep costs. This finding is not just a minor enhancement but represents a substantial leap forward in the search for economically viable catalytic solutions.
Delving deeper into the physical chemistry of the new catalyst reveals the mechanics behind its impressive performance. The researchers employed advanced methodologies including microkinetic modeling and density functional theory (DFT) calculations. These techniques were instrumental in elucidating the impact of erbium doping on the Co3O4 structure. Doping introduced more active sites and defects within the crystal lattice, which altered the Co3+/Co2+ ion ratio. This shift fosters the formation of oxygen vacancies—microscopic voids essential for accelerating the OER by facilitating the movement of reaction intermediates.
As co-author Hao Li eloquently puts it, “Imagine the catalyst like a road.” The roads are lined with lanes, and the introduction of erbium effectively broadens these lanes, allowing for a smoother and faster transport of reactants—specifically, oxygen intermediates—through the catalytic process. The findings indicate that these oxygen vacancies are largely situated in the octahedral sites of the Co3O4 structure, catalyzing the development of vital intermediates necessary for the OER.
The implications of this research extend beyond mere performance metrics. By demonstrating that a small percentage of a rare earth element can lead to significant structural and electronic optimization, the study provides a blueprint for future work in catalyst design. As Wang notes, balancing electronic properties with structural considerations is key to unlocking high-performance catalysts that maintain low costs and are easily sourced.
Looking ahead, the team intends to broaden their focus onto exploring non-precious metal dopants. The aim is to further innovate within the realm of low-cost and readily available electrocatalysts, which can play an integral role in the shift toward sustainable energy solutions. As the world grapples with energy challenges, the development of such materials could pave the way for more viable and economically accessible technologies in renewable energy sectors.
The introduction of erbium-doped Co3O4 represents an important milestone in the quest for efficient oxygen evolution reactions. This innovative approach not only enhances catalytic performance but also showcases the potential for low-cost alternatives to expensive materials like iridium. As researchers continue to explore this domain, the development of sustainable, cost-effective electrocatalysts could substantially impact future energy conversion technologies, aligning with global efforts toward a greener planet.

Leave a Reply