In the constantly evolving realm of biomedical engineering, one of the most groundbreaking advancements is the development of bioprinting technology. At the forefront of this innovation, a research team led by Liheng Cai from the University of Virginia School of Engineering and Applied Science has unveiled a remarkable approach to creating human-compatible organ components on demand. This groundbreaking work, which blends science with hope for the future of medicine, seeks to bridge the gap between artificial constructs and the complexity of human tissue.
A key component of their success is the innovative technique dubbed the Digital Assembly of Spherical Particles (DASP). By utilizing this method, Cai and his doctoral student, Jinchang Zhu, can print biomaterials with mechanical properties that closely mirror those of human tissue—an impressive feat that significantly elevates the standards of bioprinting. This new paradigm represents not just a step forward but a leap that could redefine the landscape of tissue engineering and regenerative medicine.
The Technology Behind DASP
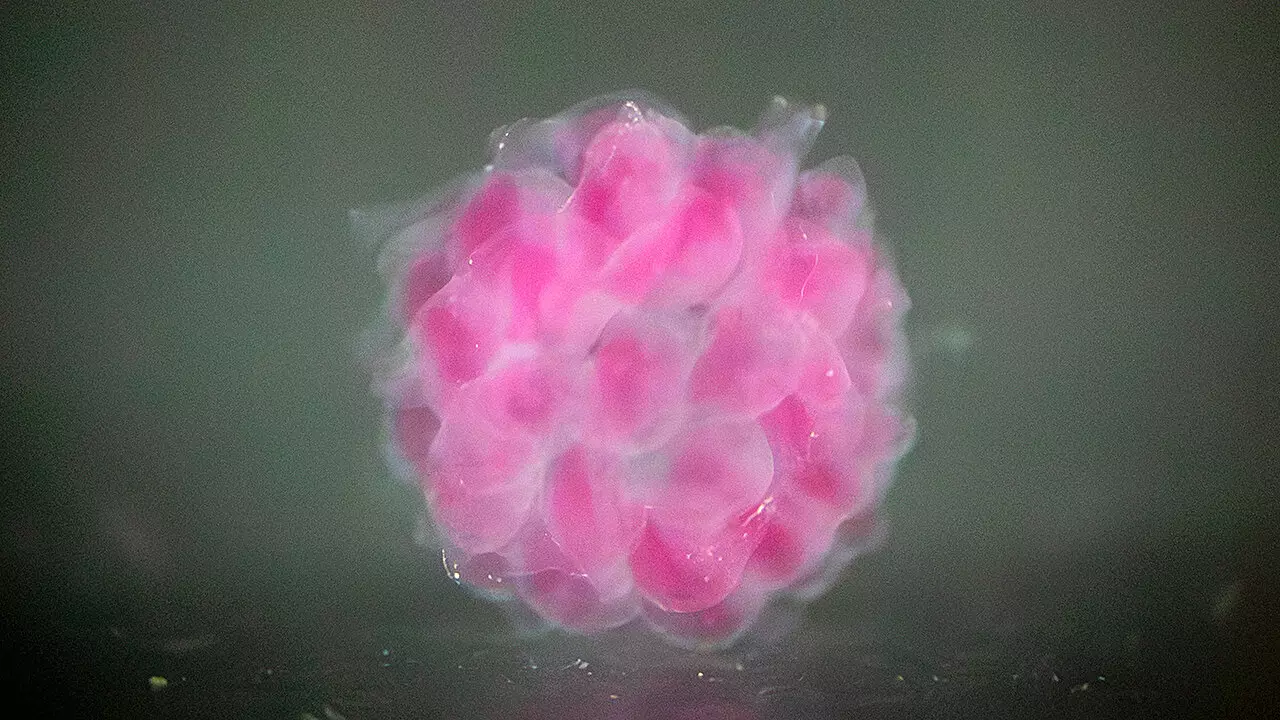
DASP functions through an intricate process where biomaterial particles are deposited into a water-based supporting matrix, allowing the formation of complex 3D structures that create ideal environments for cell growth. This innovative approach utilizes “voxels,” which are the three-dimensional equivalents of pixels, to construct these intricate structures. In doing so, the researchers have achieved a level of precision in creating what Zhu describes as the “first functional voxel,” paving the way for the potential to develop organoids—miniature organs that can model disease processes.
What’s remarkable about the materials they have developed is their ability to encapsulate human cells within polymer hydrogels designed to closely resemble the biological architecture of human tissues. Through meticulous manipulation of single-molecule monomers, the team has created a versatile double-network hydrogel that maintains both strength and biocompatibility—essential requirements for any material meant to interact with living cells. Compared to conventional hydrogel bio-inks that often exhibited toxicity, Cai and Zhu’s advancements promise a safer and more efficient pathway for organ regeneration.
From Concept to Action: The Evolution of DASP
To fully appreciate the significance of DASP, one must consider the groundwork laid by the research team prior to their latest achievements. Their previous efforts, documented in 2021, confirmed the concept of utilizing biomaterial voxels and demonstrated a printed material capable of functioning like a pancreas. However, the limitations were evident; rigid hydrogels lacked the tunability required for replicating the soft, flexible nature of human tissues.
With the advent of DASP 2.0, the researchers have transformed their initial vision into a dynamic reality. Utilizing “click chemistry” for rapid molecular cross-linking, the latest iteration of their technology allows for a seamless transition from liquid to a resilient gel structure within an astonishingly short time frame. The development of a multichannel nozzle further enhances the process by enabling the mixing of hydrogel components on-the-fly, thus preserving the integrity of the biomaterials as they are printed.
The Implications for Future Healthcare
Cai and Zhu’s work stands on the precipice of revolutionizing various aspects of healthcare. The bioprinting of functional organs and tissues could drastically alter the landscape of organ transplantation, significantly reducing wait times and eliminating transplant rejection issues. Furthermore, the ability to model diseases in vitro using printed organoids could expedite drug discovery and development, leading to more effective treatments and cures.
As the researchers continue to refine their methods, the implications of DASP reach far beyond mere organ creation. In the realms of disease modeling and drug screening, the potential for personalized medicine advances, allowing treatments to be better tailored to individual patients. As Cai notes, the challenges they face today represent not just technical hurdles but vital questions about the future capabilities of bioprinting technology in medicine.
The journey toward bioprinted organs and tissue equivalents is still underway, but with advancements like those made by Cai and Zhu, the future of regenerative medicine appears bright and full of possibility. We stand on the brink of what may well be a pivotal moment in our understanding of biology, facilitating breakthroughs that can save countless lives and redefine healthcare as we know it.

Leave a Reply