Hydrogen has always caught the attention of scientists and energy advocates alike, primarily due to its abundant availability and lack of carbon emissions when used for energy generation. As the clean energy movement accelerates, hydrogen stands out as a key player in achieving a sustainable future. However, while hydrogen itself is plentiful in the universe, it scarcely exists in its elemental form. Instead, it is predominantly locked within various chemical compounds such as ammonia and metal hydrides. Among these compounds, ammonia has emerged as one of the most viable hydrogen carriers, possessing a high hydrogen content that constitutes 17.6% of its mass, an advantageous feature especially as energy demands increase.
The apparent benefits of ammonia as a hydrogen source are somewhat overshadowed by the substantial challenges associated with its decomposition. Traditional methods require extremely high temperatures—typically above 773K—making it impractical for on-demand hydrogen generation needed in fuel cells or combustion engines. Moreover, inconsistencies in conversion rates can impede the effectiveness of ammonia as a hydrogen carrier. Such challenges highlight the urgent need for innovative methodologies that can make hydrogen extraction from ammonia more accessible and efficient.
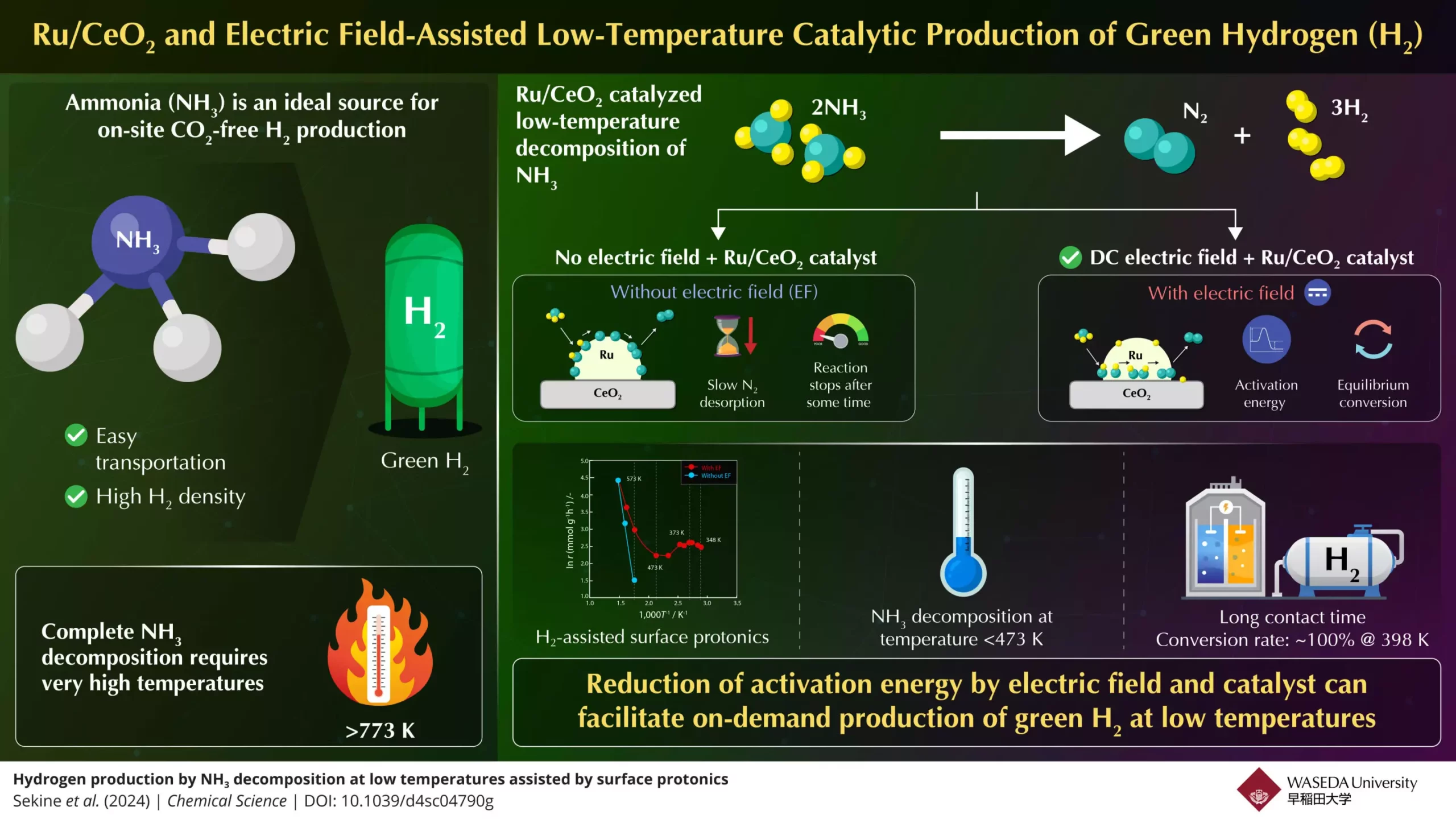
Enter Professor Yasushi Sekine and his team from Japan’s Waseda University, who have taken substantial steps to overcome these temperature barriers. Collaborating with experts from Yanmar Holdings, they have developed a novel approach aimed at facilitating the breakdown of ammonia into hydrogen at much lower temperatures. Their research, recently published in *Chemical Science*, centers around a high-performance catalyst made from ruthenium and cerium dioxide (Ru/CeO2) enhanced by the application of a direct current (DC) electric field. This innovative process not only increases the rate of hydrogen production but does so at temperatures below 473 K—a remarkable achievement in the field of energy conversion.
Professor Sekine’s team meticulously analyzed the chemical reactions involved in breaking down ammonia. They discovered that the rate-limiting step for hydrogen gas production lies in the desorption of nitrogen at lower temperatures and the dissociation of N–H bonds at higher temperatures. By employing an electric field during the catalytic process, the researchers enhanced proton conduction on the catalyst’s surface, effectively lowering the activation energy required for the reaction to occur. The result was a significant reduction in the thermal demand of the ammonia decomposition process, thus facilitating efficient hydrogen output.
Through this pioneering research, Sekine’s team successfully demonstrated that under optimal conditions, using their electric field-assisted method, they could achieve a staggering 100% conversion rate at a mere 398 K. This surpassed traditional limits and opened a pathway toward generating green hydrogen with unprecedented efficiency. The improvement was attributed not simply to the catalyst’s composition, but critically to the influence of electric field-induced protonics, which promotes the movement of protons across the catalyst surface.
This breakthrough holds immense potential for expanding the role of hydrogen as a clean alternative fuel. With the new low-temperature ammonia decomposition technique, efficiency increases significantly, making on-demand hydrogen synthesis not just a theoretical possibility but a practical reality. The ease of utilizing ammonia, which can be stored and transported more conveniently compared to hydrogen gas, positions it as a prime candidate for large-scale hydrogen production.
Ultimately, the research conducted by Sekine and his team signifies a monumental advancement in the hydrogen energy landscape. Their work paves the way for more widespread adoption of hydrogen as a clean energy source, bringing us closer to a future where fossil fuels are a thing of the past. As global energy needs continue to rise and the quest for sustainability intensifies, innovations such as these will be pivotal in addressing the world’s energy challenges and moving towards a greener planet.

Leave a Reply