As the world grapples with an escalating energy crisis, the need for sustainable and efficient energy alternatives has never been more urgent. Hydrogen energy, characterized by its green nature and high calorific value, stands out as a viable solution. However, despite its promise, several technological hurdles remain in making hydrogen production more efficient. A significant challenge is the slow kinetics of the oxygen evolution reaction (OER), which is a critical step in the electrochemical water-splitting process used to produce hydrogen. Addressing this challenge through the development of effective catalysts is essential for the advancement of hydrogen energy technology.
Catalysts play a pivotal role in enhancing the efficiency of chemical reactions. In the context of OER, catalysts facilitate the conversion of water into hydrogen and oxygen, thus reducing the energy input required for the reaction. The performance of these catalysts is often contingent upon their structure and composition. Recent advances in the field of catalysis have led to a growing interest in single-atom catalysts (SACs), which offer a unique approach to maximizing reaction efficiency. A standout among these innovations is the high-density Ir single-atom catalyst, a topic recently explored by researchers led by Prof. Bao Jun at the University of Science and Technology of China (USTC).
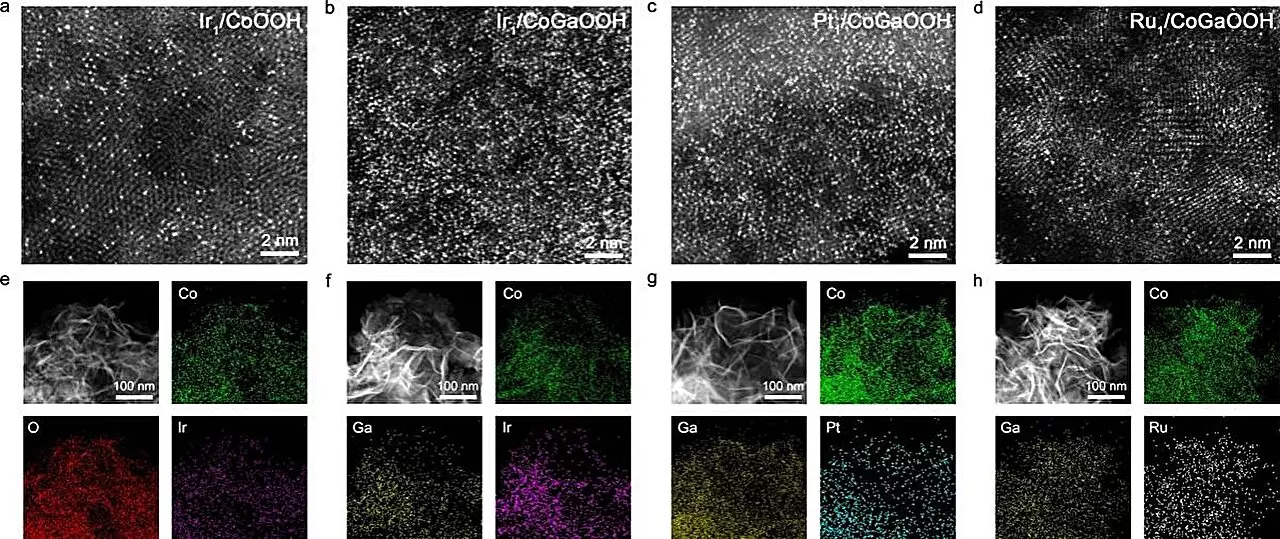
High-Density Single-Atom Catalysts: A Breakthrough
The research conducted by Prof. Bao Jun’s team highlights the remarkable potential of cobalt-based, oxide-supported high-density Ir single-atom catalysts. Their findings reveal that these catalysts exhibit superior performance in electrochemical OER. This performance is notably enhanced by the interactions occurring between nearby single atoms, a phenomenon termed neighboring synergy. As the density of these single atoms increases, they become more closely spaced, which in turn optimizes their catalytic behavior by improving the adsorption of reaction intermediates.
The team’s innovative strategy involved the incorporation of gallium (Ga) atoms into the cobalt-based oxide lattice. This modification adjusted the electronic structure of the anchoring sites for the Ir single atoms. The enhanced bonding strength between the oxygen defect sites and the precursors effectively led to the construction of high-density SACs. This meticulous development process is crucial, as it opens avenues for maximizing catalyst efficiency.
The research team conducted several evaluations to determine the efficiency of these novel catalysts. The high-density Ir single-atom catalyst, known as Nei-Ir1/CoGaOOH, demonstrated exceptional performance, showcasing a low overpotential of 170 mV at a current density of 10 mA cm-2. Furthermore, it exhibited impressive durability, maintaining stability for over 2000 hours. Notably, the catalyst also performed reliably at a higher current density of 1 A cm-2 in alkaline electrolyte for an extended period of over 50 hours.
These findings are crucial as they demonstrate not just the immediate efficacy of the catalysts but also their long-term viability in practical applications. In situ Raman spectroscopy was employed to confirm that the catalyst maintained its structural integrity throughout the OER process, further solidifying its promise as a reliable catalyst.
Unraveling the Mechanism of Enhanced Performance
While the performance of the high-density Ir single-atom catalysts was commendable, the underlying mechanisms driving this enhancement were further scrutinized. Interestingly, the enhancement in performance was not simply due to modifications in the electronic structure of the active sites. Instead, it stemmed primarily from the neighboring synergetic interactions among the high-density Ir single atoms. These interactions stabilize the *OOH intermediates through additional hydrogen bonding, leading to a reduction in the reaction’s energy barrier and ultimately enhancing the catalyst’s overall performance.
The exploration of high-density single-atom catalysts, particularly cobalt-based catalysts featuring Ir, signals a transformative step toward advancing hydrogen production technologies. As researchers continue to uncover the complexities of catalytic interactions, there lies immense potential for developing more efficient and sustainable energy solutions. This research not only highlights the mechanisms behind catalyst efficiency but also paves the way for future innovations in electrochemical systems, ensuring that hydrogen energy can play a significant role in addressing the global energy crisis. With continued experimentation and development, these insights could lead to breakthroughs that redefine our energy landscape in the near future.

Leave a Reply