Recent advancements in chemical synthesis have unveiled a compelling innovation, primarily driven by the desire to streamline the production of ethers—a critical class of compounds prominently used in pharmaceuticals, consumer goods, and personal care items. Researchers at the University of Illinois Urbana-Champaign, under the guidance of Professor M. Christina White, have engineered a game-changing catalyst that mimics natural enzymes. This novel catalyst not only enhances the efficiency of ether synthesis but also enables the formation of previously unattainable ethers.
Ethers are ubiquitous in modern life, found in a wide range of products from medicines to flavorings. Ethereal compounds contribute essential functionalities that help define the characteristics of various consumer goods. Despite their prevalence, traditional methods of ether synthesis can be cumbersome, often requiring the use of significant quantities of reactants and yielding a complex mix of byproducts. This complexity can lead to extensive purification processes, ultimately complicating the manufacturing of these essential compounds.
In standard synthesis methods, the production of ethers typically involves the activation of an alcohol by removing a proton. This activation renders the alcohol reactive but also produces a variety of unwanted byproducts, necessitating labor-intensive separation techniques to isolate the desired ether. Furthermore, conventional approaches often result in low yields and require substantial amounts of starting materials, a significant barrier in developing complex molecules that hold significant value in various industries.
Graduate student Sven Kaster emphasized the motivation behind this research: “We aimed to circumvent the need for drastic activations of our reactants and to utilize minimal quantities.” As chemists sought a more elegant solution, they turned towards the principles of nature, recognizing the efficiency exhibited by biological systems in catalyzing reactions.
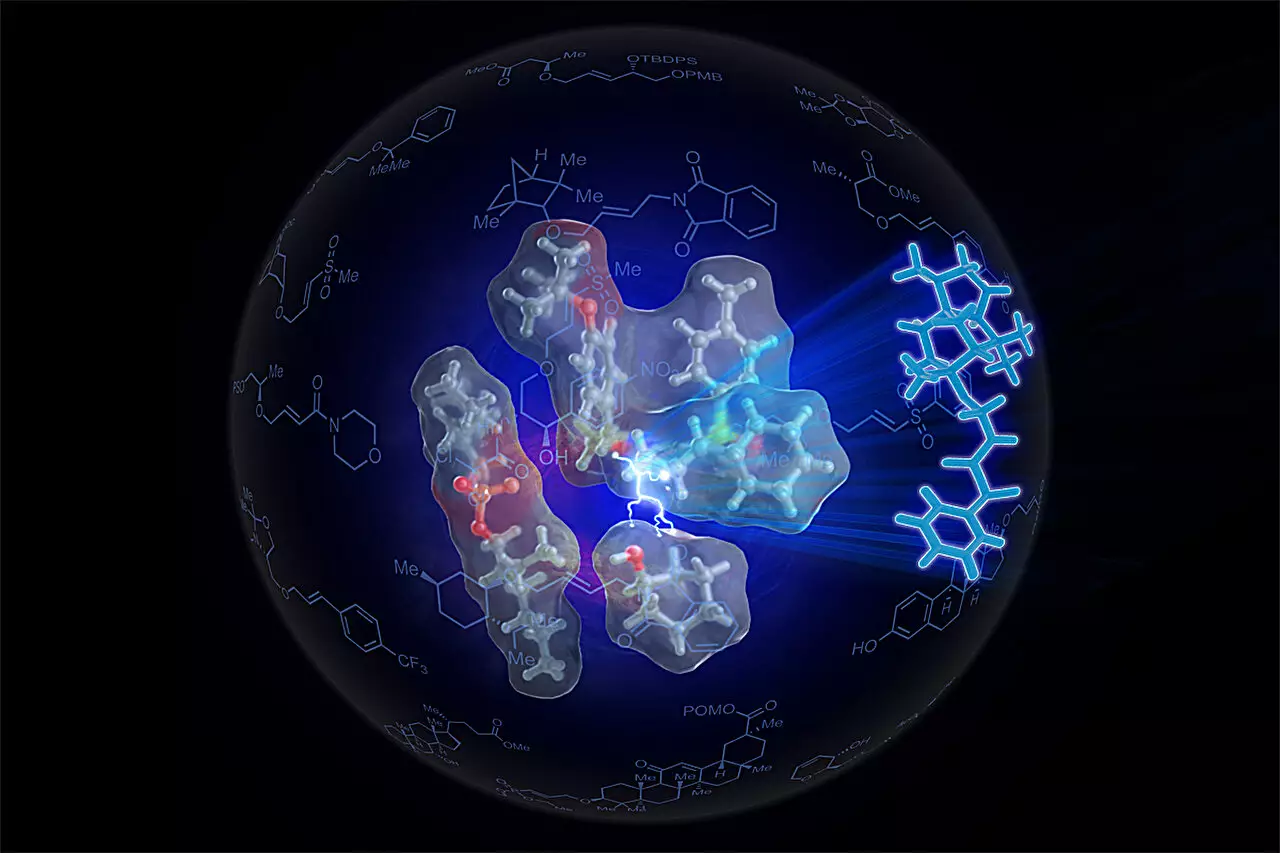
The researchers found their answer in the intricate world of enzymatic reactions. Enzymes are biological catalysts renowned for their ability to facilitate and accelerate biochemical reactions with remarkable precision. They achieve this by positioning reactants optimally and ensuring they are suitably oriented for interaction. White and her team aimed to replicate this concept through the development of a self-assembling catalyst that employs palladium to enable the necessary reactions.
This innovative catalyst has been aptly named SOX, and its groundbreaking capabilities stem from its unique design. By altering the catalyst’s geometry and electronic characteristics, the researchers successfully achieved the alignment of functional groups required to generate ethers. Remarkably, the catalyst can convert alkenes and alcohols with minimal activation and under gentle conditions, a stark departure from prior methods.
The team progressed to produce a refined version of the SOX catalyst, dubbed Sven-SOX, specifically engineered to optimize ether synthesis. This self-assembling construct enhanced the interaction between alkene and alcohol, enabling the formation of ethers through an elegant mechanism—much like two individuals joining hands. “To effectively hold hands, you must be closely positioned and facing one another appropriately,” explained White, drawing parallels between human interaction and chemical reactions.
The findings indicate that the Sven-SOX catalyst excelled across a diverse range of ether-producing reactions. In their research, the team successfully synthesized over 130 distinct ethers, some of which contained complex molecular architectures that posed difficulties for traditional synthesis methods. This breakthrough not only signifies advancements in chemical engineering but also offers newfound opportunities for innovation in creating compounds with varied chemical functionalities.
The implications of this research extend well beyond ethers. The techniques developed by White and her team provide insights into how small molecule catalysts can be harnessed to replicate enzymatic functions for broader applications in chemical synthesis. Their ongoing research aims to explore additional small-molecule catalysts that can facilitate the creation of other chemical classes, harnessing the same principles of proximity and orientation that have proven effective in synthesizing ethers.
Reflecting on their findings, Kaster noted, “The versatility of our approach opens doors to ethers that may possess entirely new or useful properties.” By maintaining milder reaction conditions, this method accommodates sensitive functional groups, which could facilitate the synthesis of more complex compounds while reducing material waste.
The work being undertaken at the University of Illinois Urbana-Champaign encapsulates the vital intersection of chemistry and biology, showcasing the potential of nature-inspired solutions to advance synthetic methodologies in chemistry. This innovative approach heralds a brighter future for the field, demonstrating how a deep understanding of natural processes can lead to the development of efficient and effective chemical synthesis techniques.

Leave a Reply