In a significant advancement for analytical chemistry, researchers at Oak Ridge National Laboratory (ORNL) have successfully combined two sophisticated techniques to detect fluorine and various isotopes of uranium within a single particle simultaneously. This groundbreaking achievement holds considerable implications for the field of nuclear materials inspection, particularly for the International Atomic Energy Agency (IAEA). By identifying both elements concurrently, inspectors could gain critical insights into the potential applications of nuclear materials, whether for peaceful or nefarious purposes.
Published in the esteemed Journal of the American Chemical Society, the study marks a pivotal moment in the ability to characterize individual particles concerning their isotopic, elemental, and chemical compositions. The identification of isotopes—Variants of chemical elements that share the same proton count but differ in neutron numbers—is crucial for understanding chemical processes and determining the age of materials.
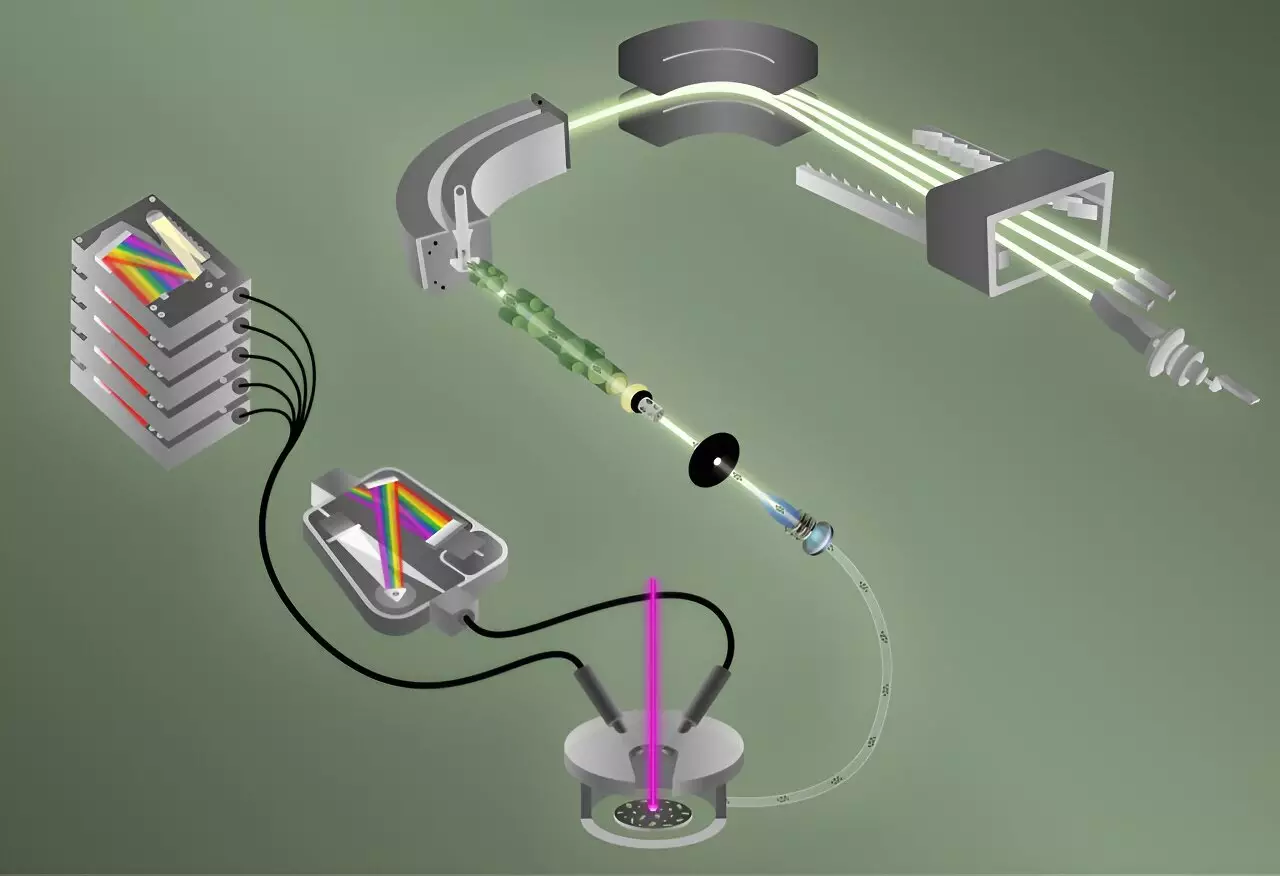
According to Benjamin Manard, the lead researcher from ORNL, traditional methods of determining isotopic ratios in single particles are labor-intensive and time-consuming. His team has innovated a means to analyze 40 particles, each comparable in size to a human red blood cell, in under five minutes—a significant reduction in the time usually required for such assessments. This new technique employs laser-induced breakdown spectroscopy (LIBS) to quickly and sensitively detect the element fluorine.
LIBS operates by vaporizing the particle sample, creating plasma when subjected to laser pulses. As the plasma cools, it emits light, which researchers measure to identify various elements present. Manard likened the process to a fireworks display, where different elements emit distinctive colors corresponding to their light wavelengths.
In conjunction with LIBS, the team incorporated a mass spectrometry technique called laser ablation multicollector inductively coupled plasma mass spectrometry (ICP-MS), which is essential for DNA isotopic characterization. The combination of these techniques offers a comprehensive platform that achieves simultaneous integration of fluorine detection and uranium isotopic analysis.
The presence of uranyl fluoride—an inorganic compound of uranium, oxygen, and fluorine—can reveal crucial information about nuclear processes. Brian Ticknor, a co-author of the study, asserts that detecting both uranium and fluorine within the same particle carries significant importance for nuclear nonproliferation efforts. By evaluating the ratio of these substances, analysts can inferred data regarding the origins of the material, its production processes, and the timeframe during which it was formed.
The implications of this research extend well beyond national security considerations. The innovative application of these detection technologies holds promise in several diverse fields including advanced nuclear reactor fuel design, next-generation battery development, and even environmental science, particularly in elucidating the behavior and impact of microplastics.
A key challenge that the researchers faced was the difficulty of integrating LIBS and ICP-MS techniques. The former utilizes a unique method of identifying elements directly in gaseous plasma, while the latter relies on generating positive ions to perform accurate mass spectrometry. Fluorine molecules tend to have a strong inclination towards a negative charge, complicating their analysis using traditional ICP-MS methods.
By integrating these techniques, ORNL’s team has taken a pioneering step—although counterparts have previously applied LIBS and ICP-MS independently, no one had achieved simultaneous measurements in a multicollector configuration. The multidisciplinary collaboration facilitated by ORNL’s Ultra-trace Forensic Science Center was essential in overcoming these challenges.
The researchers envision numerous applications for their enhanced analytical capabilities. They initially employed this technology to investigate the distribution of fluorine in shark teeth samples obtained from the Georgia Aquarium—a project revealing not only current but also historical environmental conditions based on the fluoride concentration in tooth enamel. The need for rapid particle analysis is crucial in diverse areas, with an emphasis on potentially extending this technology to analyze even more complex compounds related to nuclear processes.
With plans in place to identify other uranium compounds, such as those containing chlorine, researchers are set on improving the already impressive capabilities of their innovative methodology. The joint techniques hold substantial promise for revolutionizing our approach to particle analysis across varied scientific fields, spotlighting the profound intersection between analytical chemistry and real-world applications.
The collaboration between advanced spectroscopic methods has set a new standard for the analysis of nuclear materials. As the research progresses, the benefits of understanding isotopic compositions and elemental distributions will undoubtedly reach far beyond nuclear safeguards, potentially influencing multiple branches of science and industry.

Leave a Reply