The pharmaceutical industry is a bastion of innovation, yet its inherent challenges are staggering. Developing a new drug is not just a journey; it’s often a minefield characterized by years of rigorous research, vast financial outlay—often amounting to hundreds of millions of dollars—and, unfortunately, a dismal success rate. Indeed, more than 90% of drug candidates falter at the crossroads known as clinical trials, and many others do not even reach that stage. Among the many culprits for this failure, safety concerns overwhelmingly stand out. The landscape is transforming, however, as artificial intelligence (AI) emerges as a beacon of hope to streamline the process.
AI Models: A New Frontier in Toxicology
Researchers at the Broad Institute of MIT and Harvard have taken a bold step forward by developing machine learning models capable of predicting a drug’s toxicological effects before they ever enter human trials. This approach significantly alters the drug development paradigm, allowing scientists to harness predictive analytics for preemptive insights. Srijit Seal, a visiting scholar at the Broad’s Imaging Platform, has been at the forefront, training machine learning models that probe chemical and structural features to assess their potential for toxicity. This isn’t just a numeric analysis; it’s about delving into the very essence of molecular biology to predict how drug compounds could interact with human biology.
The implications of this work are profound. By achieving a nuanced understanding of how various drugs impact cellular health, pharmacokinetics, and essential organ functions—such as heart and liver health—researchers are better equipped to streamline their focus. The tools developed are not intended to serve as a full replacement for traditional laboratory experimentation but rather as an invaluable mechanism to refine the selection of candidates that warrant deeper investigation.
Machine Learning’s Uncanny Precision
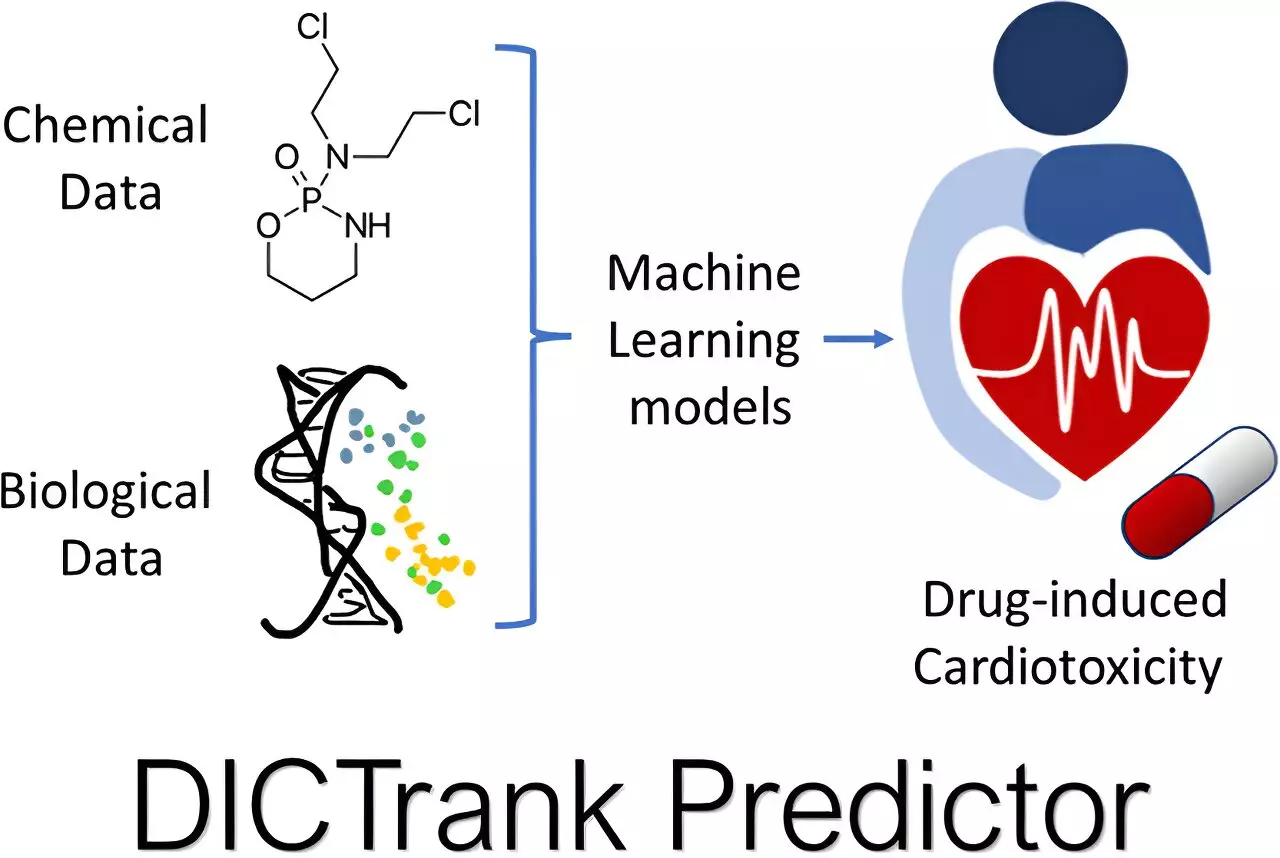
Seal’s research is reminiscent of a detective unraveling the threads of toxicity linked to chemical structures. Notably, two primary models emerged from his efforts: one predicts cardiotoxicity while the other assesses the likelihood of liver injuries. What sets these models apart is their ability to rely on structured data specifically curated by the FDA, which includes detailed lists of known drug toxicities. By combining this information with diverse chemical properties, Seal’s models effectively sift through a myriad of candidates, isolating those that exhibit favorable profiles versus those with alarming potential for adverse reactions.
This is groundbreaking not just because of accuracy; it highlights a paradigm shift in thinking. Often, similar compounds exhibit contrasting effects in humans compared to other species, leading to high rates of post-market withdrawals due to drug-induced side effects. Seal’s research has, therefore, created a predictive predictor that can operate as a safety net before products even reach human testing—the DICTrank Predictor and the DILIPredictor stand as twin pillars of this analytical revolution.
The Evolving Nature of Pharmacokinetics
Pharmacokinetics, referring to the journey of drugs through the body—how they are absorbed, distributed, metabolized, and cleared—is equally critical to determining drug efficacy and safety. The traditional approach to pharmacokinetic modeling is often cumbersome, necessitating extensive resources and time. AI’s capacity to rapidly model these interactions opens new doors for efficiency. For developers, the concept of “failing faster” is not merely catchy jargon; it represents a pragmatic approach that allows researchers to spend time refining only those candidates that demonstrate promise.
In this context, Seal’s collaboration on pharmacokinetic modeling seeks to juxtapose new predictive models against existing industry standards, helping researchers identify faster pathways to discovery. This innovative approach creates feedback loops crucial for ensuring that drug designs align with human biological responses rather than dominating laboratory outcomes.
Biological Insights Through Imagery and Data
Delving further into drug toxicity requires insights that go beyond mere prediction of outcomes. Understanding the precise mechanisms by which compounds affect cell health becomes essential. Recognizing this gap, Seal adapted CellProfiler—an open-source imaging software—to provide a framework for identifying cellular morphological features. However, as Seal discovered, translating these intricate data points into actionable biological insights was often problematic for researchers.
To bridge this divide, he developed BioMorph, a model that integrates visual data from CellProfiler with additional parameters related to cell health. This platform assists scientists in correlating drug compounds to specific cellular responses, providing them not only with predictive power but also a detailed understanding of why certain compounds behave the way they do biologically. BioMorph has successfully demonstrated its capability by matching compounds with affected cellular features, offering a rich narrative about drug mechanisms.
The remarkable advancements made by researchers like Seal signal an exciting shift in pharmaceutical development frameworks. Enhanced predictive models represent more than technological progress—they generate a revolution in the way researchers explore drug toxicity and efficacy, setting the stage for a new era of safer, more effective therapies. The marriage between machine learning and drug discovery is still in its infancy, yet the implications are already monumental, promising a future where drug discoveries are not just made—they are designed with foresight and safety in mind.

Leave a Reply