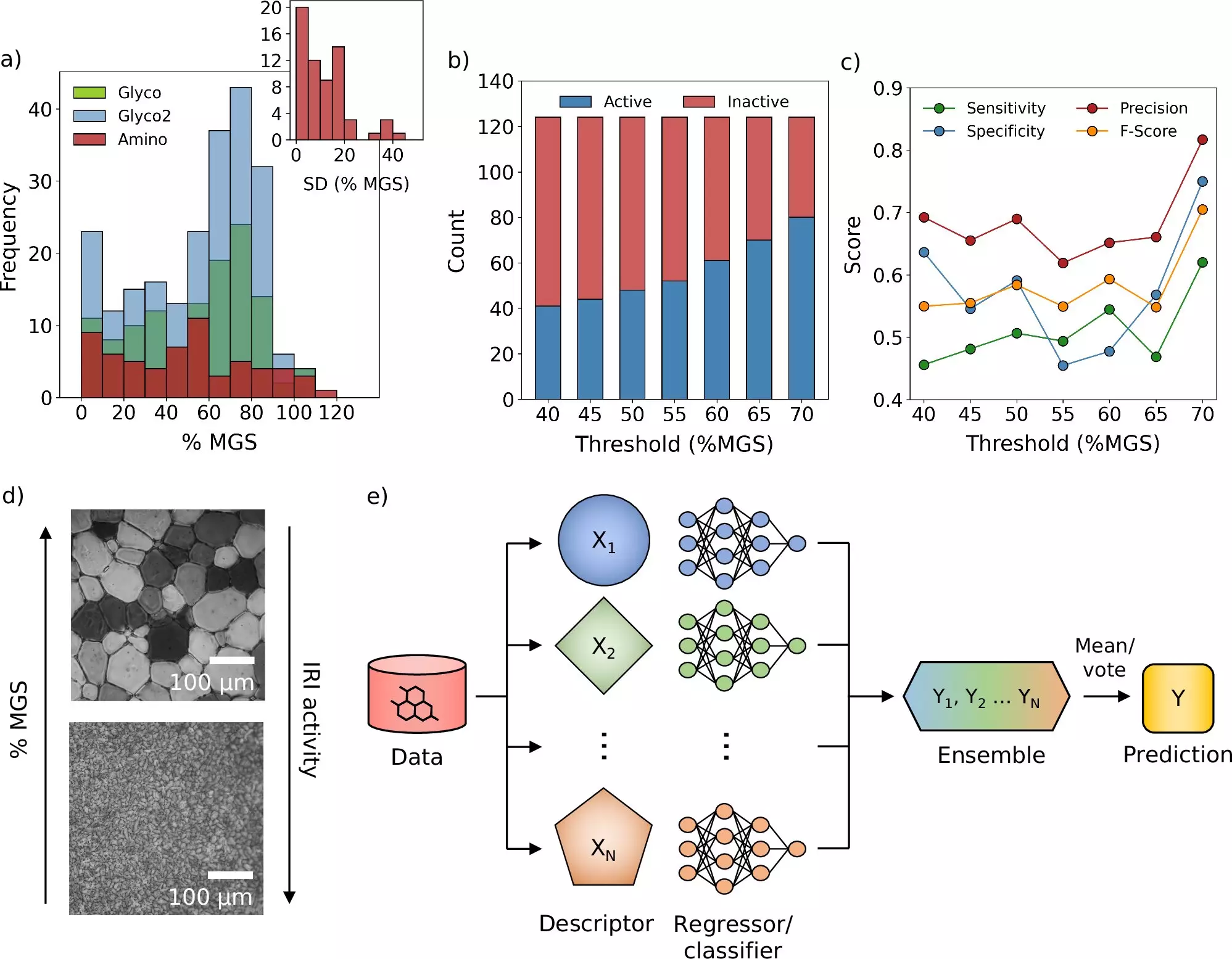
The preservation of biological materials is a cornerstone in the fields of medicine and biotechnology. Treatments needed in critical situations, such as vaccines, blood donations, and various cellular therapies, often necessitate rapid freezing methods to maintain their functional integrity. This process, known as cryopreservation, is hindered by a significant challenge: the formation of ice crystals during freezing and thawing. The presence of these ice crystals can damage cellular structures and diminish the efficacy of therapeutic agents. Therefore, scientists have long sought effective solutions, particularly through the use of cryoprotectants—substances that safeguard biological materials against the detrimental effects of ice.
Recently, a research collaboration between the Universities of Warwick and Manchester has ushered in a new era for cryopreservation practices. By deploying a state-of-the-art computational framework that utilizes machine learning and molecular simulations, scientists can now virtually screen an expansive library of compounds to identify potential cryoprotectants. Published in the esteemed journal Nature Communications, this innovative framework supplements traditional experimental methodologies with a data-driven approach that could revolutionize how new cryoprotectants are discovered.
As highlighted by Prof. Gabriele Sosso from Warwick, machine learning represents just one tool in a larger toolkit aimed at enhancing scientific research. Its integration with experimental validation has proven crucial. This collaboration of computational methods with lab work embodies the modern scientific process, where technology augments human capacity rather than replacing it.
Breakthroughs in Ice Crystal Prevention
One of the most promising outcomes of this research is the successful identification of a novel molecule that effectively inhibits ice crystal growth. Traditional cryoprotectants may protect cellular integrity, but they fail at preventing the formation of ice crystals, which remains an obstacle during both the freezing and subsequent thawing of tissues. With the addition of this new molecule, researchers aim to improve the efficacy of cryopreservation significantly, thereby broadening the applications of preserved biological materials.
Dr. Matt Warren, the Ph.D. student leading this groundbreaking project, emphasized the transformative potential of machine learning in scientific research. The ability to predict the efficacy of cryoprotectants based on extensive computational analysis can expedite the process of discovery substantially. This shift allows experts to redirect their efforts toward more complex and nuanced challenges within the field, rather than becoming bogged down by repetitive experimental protocols.
Enhancing Blood Cryopreservation Techniques
The team’s discovery not only opens avenues for new cryoprotective materials but also demonstrates immediate practical applications. In experiments involving blood storage, incorporating the new molecule was shown to reduce the quantity of conventional cryoprotectants required. This enhancement could streamline the blood washing process post-freezing, resulting in faster transfusion times—a critical improvement in emergency medical situations where time is of the essence.
This practical application underscores the significance of the research, confirming that innovative methodologies in cryopreservation not only advance theoretical knowledge but also foster real-world medical applications that can save lives.
As the research progresses, there lies an exciting potential to repurpose existing molecules that possess characteristics capable of slowing or completely halting ice crystal formation. Prof. Matthew Gibson of the University of Manchester noted the collaborative effort as a paradigm shift, stating that the computer model illuminated potential avenues for exploration he had previously overlooked after years of conventional experimentation.
The advancements achieved through integrating machine learning and molecular simulations reflect a broader trend in scientific research, where interdisciplinary approaches can yield breakthroughs that were once deemed unattainable. As more researchers adopt these advanced computational frameworks in various scientific domains, the ripple effects may positively transform research methodologies and practical applications across numerous fields.
The fusion of machine learning with molecular analysis signifies a pivotal leap in cryopreservation technology. The newfound ability to explore vast libraries of compounds efficiently represents a paradigm shift that could redefine standards in medical storage and treatment efficacy. As ongoing research continues to explore these pathways, the advancements herald a promising future where therapies can be preserved and utilized effectively around the globe, ultimately enhancing patient care and survival rates in critical health scenarios.

Leave a Reply