Recent advancements in carbon capture technology have been promising, yet significant challenges remain in transforming carbon dioxide (CO2) into useful industrial products. A recent study from the University of Twente, spearheaded by researcher Georgios Katsoukis, delves into the pivotal role that the chemical environment surrounding copper electrodes plays in influencing the conversion process of CO2 into formate. This innovative research is pivotal for enhancing the diagnostic tools and mechanisms available to optimize CO2 reduction processes.
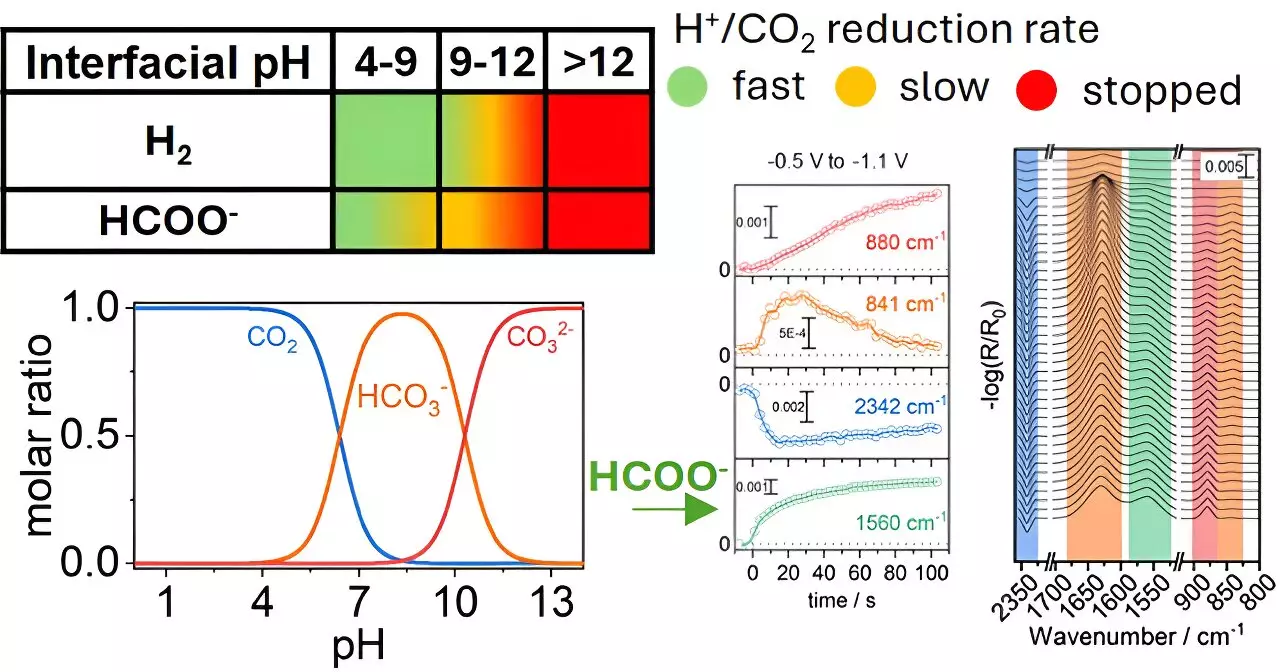
The ability to convert CO2 into more useful forms like formate is not only a critical challenge but also an opportunity in the quest for a sustainable circular economy. The interdisciplinary approach taken by Katsoukis and his team sheds light on an often-overlooked aspect of chemical reactions— the influence of local conditions at the electrode surface. By adjusting the pH levels near the copper electrodes, the researchers were able to gain insights into how these alterations significantly affect the efficiency and speed of the gas conversion process.
Such findings point to an urgent need to rethink the traditional methodologies employed in catalyst optimization. Instead of solely focusing on the catalyst materials themselves, the importance of surrounding chemical environments is laid bare. The study argues that the traditional scientific perspective may have been overly narrow, underestimating the role that external conditions play in reaction processes.
Selectivity in CO2 reduction remains one of the significant hurdles faced by researchers. Multiple products can form under varying reaction conditions, complicating the pathway toward efficiency. The breakthrough research at the University of Twente underscores that by optimizing local chemical conditions, it may be possible to favor the conversion towards a single desired product, such as formate, which has a wide range of industrial applications.
The implications are significant; a tailored chemical environment not only enhances selectivity but potentially extends the operational lifespan of copper electrodes, thereby improving the overall effectiveness and cost-efficiency of CO2 conversion technologies.
Blueprint for Future Research
The findings published in ACS Catalysis mark a pivotal moment for future CO2 reduction research. This study serves as a foundation for developing innovative technologies that are not only more selective but also more efficient in their conversion processes. As specialists aim to balance the dual challenges of selectivity and efficiency, the emphasis on optimizing the chemical environment around catalysts could lead to groundbreaking developments.
Moving forward, embracing a broader perspective that incorporates the interaction between catalysts and their chemical environments may forge the path toward practical solutions for repurposing CO2. This multifaceted approach remains essential in pursuing not just incremental improvements but transformative changes that will ultimately support efforts in fostering a sustainable and circular economy.

Leave a Reply