In an age marked by environmental concerns and a push for sustainable methods in industrial processes, researchers at the University of Illinois Urbana-Champaign have made significant strides in the field of electrochemical separation. This innovative technique employs a specially engineered polymer that selectively extracts specific substances from mixtures when activated by an electric current. The groundbreaking work, recently published in the journal JACS Au, highlights the immense potential of this technology to minimize waste in chemical separation processes—a major challenge plaguing traditional methods.
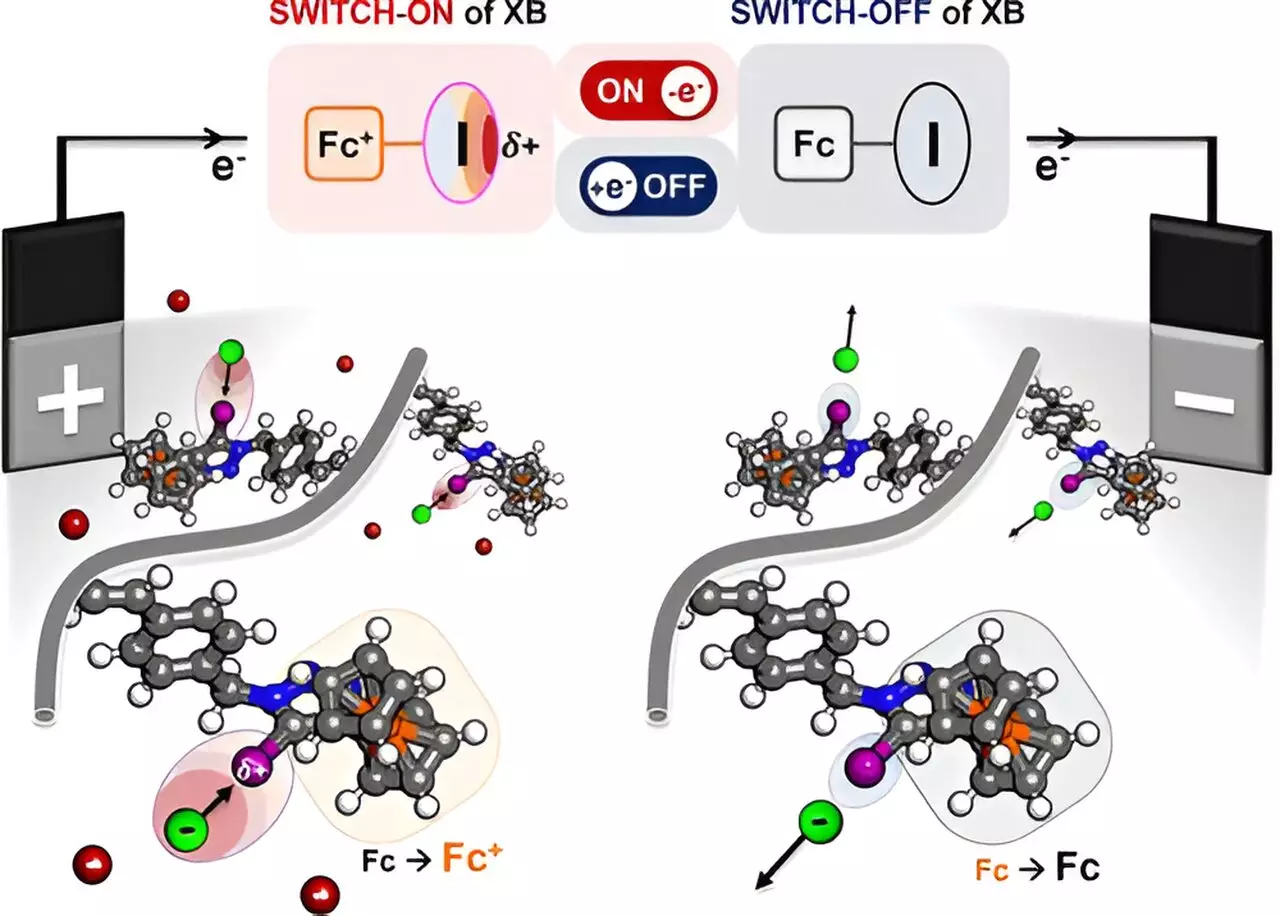
At the heart of this advancement lies the concept of halogen bonding, which entails a distinctive chemical interaction whereby a halogen atom can form strong attractions with certain molecules. The researchers adeptly harnessed this interaction by engineering a polymer featuring a redox-responsive halogen donor. Electric current enhances the polymer’s ability to modulate the charge density on the halogen atom, thereby enabling it to attract selective ions and organic molecules, including halides and oxyanions.
This clever design acts much like a molecular sponge, allowing the polymer to selectively “soak up” the desired constituents from a solution while leaving behind unwanted materials. With potential applications spanning pharmaceuticals to complex chemical syntheses, this advance could transform how manufacturers approach material separation.
Chemical separation techniques such as thermal distillation or membrane filtration are prevalent in industry but are often fraught with inefficiencies and waste. These traditional methods can generate copious amounts of byproducts or require extensive energy inputs. Conversely, the electrochemical approach being explored by this research team not only offers a way to reduce waste but also capitalizes on sustainable electricity sources, potentially heightening its appeal in eco-conscious industries. Current methods that utilize electrochemical mechanisms tend to be non-selective, pulling in various substances without regard to the desired outcome. The Illinois team’s method aims to change that narrative by introducing the concept of selectivity through electrical activation.
The research’s uniqueness lies in the development of redox-active polymers, specifically designed to alter their bonding capabilities upon electrical stimulation. The polymer, which incorporates iodine and the redox agent ferrocene, switches the attraction mechanism on and off—allowing researchers to control which ions are drawn into the polymer’s structure. This innovation is monumental as the selective capturing of ions is crucial for maintaining purity during separation processes, especially in sensitive applications like pharmaceuticals where contaminants can lead to significant complications.
The researchers, led by Professor Xiao Su, have successfully validated their approach through rigorous testing, employing methods such as nuclear magnetic resonance and Raman scattering to confirm the operational capabilities of the polymer. This foundational work provides a new avenue of exploration in the intersection of chemistry and electrical engineering.
Future Directions and Implications
Looking ahead, the implications of this research extend beyond academia. The team is poised to further refine their methods and engage in scaling-up strategies that will enable real-world applications. Future research will explore designing a continuous electrosorption system while assessing the performance of existing methods in varying environmental conditions. This groundwork has the potential to lead to significant advances in how industries manage chemical separations, driving both efficiency and sustainability in manufacturing processes.
Overall, the work on selective electrochemical separation marks a promising step toward greener industrial practices. As more stakeholders demand environmentally responsible methodologies, innovations like these could serve as a model for future technologies. The elucidation of these fundamental principles underscores the importance of interdisciplinary research in solving contemporary scientific challenges, paving the way for a more sustainable and efficient chemical-processing landscape.

Leave a Reply