The field of chemical manufacturing is on the brink of transformation thanks to innovative electrochemical techniques. In a recent advancement, researchers at Lawrence Livermore National Laboratory (LLNL) have developed a method that not only enhances energy efficiency but also promotes ecological sustainability. By utilizing thin film nickel anodes, this method represents a pivotal step towards reducing the environmental footprint of chemical production.
The use of thin film technology is critical in this new electrochemical approach. Thin films provide a uniform surface area, which is essential for studying catalytic reactions accurately. This consistency allows scientists to eliminate variables associated with uneven or porous structures, honing in on the authentic properties of the catalysts employed. According to Aditya Prajapati, a postdoctoral researcher involved in the study, this precision is vital for unraveling the complexities of chemical reactions and enhancing their efficiency.
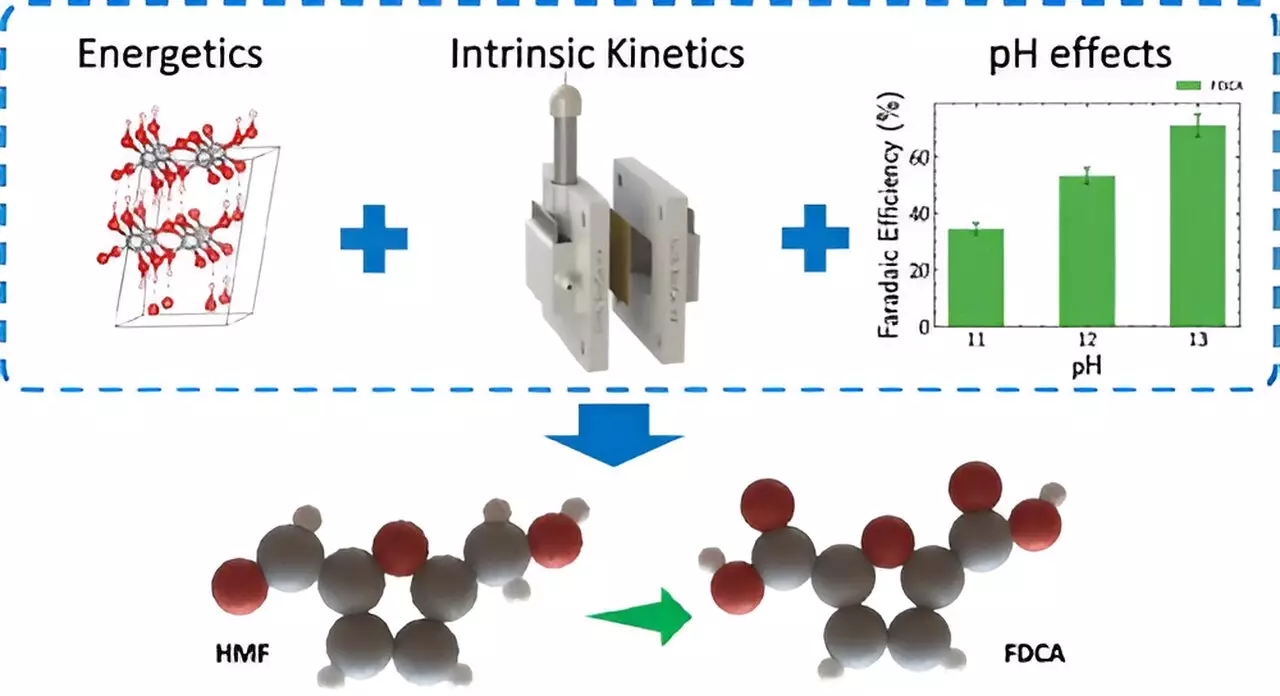
Current electrolyzer technologies face significant challenges, particularly with the oxygen evolution reaction that occurs at the anode, leading to inefficiencies in energy conversion. In a groundbreaking shift, the LLNL team discovered that substituting this reaction with biomass oxidation can reduce energy consumption by over 50%. This approach is not only innovative but also addresses the pressing issue of sustainable biomass conversion, crucial in an era where environmental concerns dominate.
The team’s research specifically highlights the conversion of 5-Hydroxymethylfurfural (HMF), a biodegradable compound sourced from biomass, into 2,5-Furandicarboxylic acid (FDCA). FDCA is rapidly gaining recognition as a key ingredient in the manufacture of sustainable plastics, such as polyethylene furanoate (PEF), a bio-based alternative to traditional petroleum-derived plastics. By channeling this electrochemical method, researchers are effectively cutting down on reliance on fossil fuels while simultaneously reducing carbon emissions.
Traditional chemical production often involves high-temperature processes accompanied by toxic byproducts, making them environmentally unfriendly. In contrast, the method developed by LLNL researchers is both cleaner and more energy-efficient. Its relevance to renewable energy sources is particularly compelling; when powered by renewable electricity, the electrochemical oxidation of HMF to FDCA could achieve a zero-carbon footprint. This advancement not only supports the notion of a circular economy but also emphasizes the potential for sustainable feedstocks in industrial applications.
This remarkable research effort underscores the importance of collaborative work among scientists across institutions, including contributions from Université de Montréal and the University of Bonn. The collective expertise harnessed in this study demonstrates a promising trend towards reimagining the future of chemical production, focusing on reduced environmental impact and increased energy efficiency.
LLNL’s breakthrough in electrochemical methods signals a new era for sustainable chemical production. As the industry faces immense pressure to lessen its environmental impact, innovations like these pave the way for a cleaner, more efficient future, reflecting a commitment to sustainability and responsible resource management.

Leave a Reply