In the quest for a more sustainable chemical industry, the pursuit of effective methods for converting biomass into valuable chemicals has garnered significant attention. Recent research from Kyushu University highlights an innovative approach utilizing microwaves to enhance the catalytic conversion of biomass into olefins—crucial precursors for a plethora of products, including plastics and pharmaceuticals. This groundbreaking study, published in the Chemical Engineering Journal, not only showcases the efficiency of this method but also posits a transformative avenue toward greener chemical processes.
Understanding the synthesis of complex organic compounds requires a firm grasp on the fundamental precursors involved. Traditional methods like naphtha reforming, while prevalent, are marred by energy inefficiencies and high carbon emissions. The shift towards alternative sources, such as waste cooking oil and microalgal oils, presents an attractive solution, but the challenge remains in developing sustainable and efficient conversion methods. The catalytic cracking process, historically reliant on zeolites, often demands extensive heating and energy input, exacerbating both operational costs and environmental detriment.
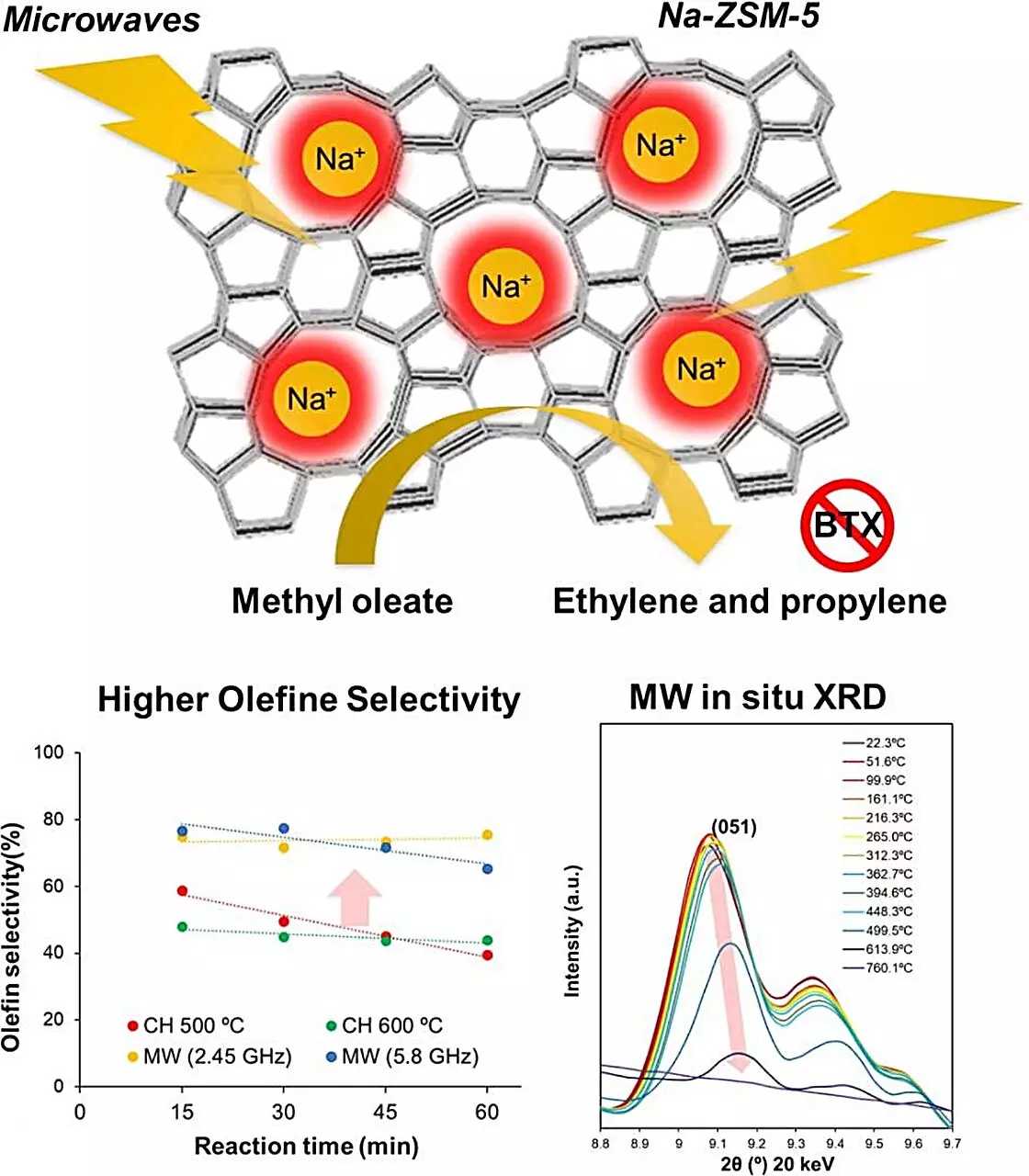
Zeolite, a naturally occurring porous material, has long been revered for its catalytic properties. However, traditional catalytic cracking processes leverage temperatures exceeding 500-600°C, leading to significant energy expenditures and the risk of coking. This study, led by Associate Professor Shuntaro Tsubaki, introduces a novel approach by pursuing microwave-assisted heating of zeolite catalysts, specifically Na-ZSM-5. The research team meticulously examined the interaction between microwaves and zeolite materials, which facilitated a more efficient transfer of energy directly to the catalyst, mitigating the energy-intensive nature of conventional methods.
Tsubaki and his team embarked on a systematic evaluation of various zeolite catalysts to identify those best suited for microwave heating. Their thorough testing process culminated in the selection of Na-ZSM-5, a sodium-modified zeolite known for its robustness in catalysis. To quantify its performance, the researchers conducted experiments converting methyl oleate into olefins, comparing microwave heating against traditional methods. The results were striking: not only did microwave-assisted Na-ZSM-5 show superior conversion rates, achieving a remarkable reduction in carbon dioxide emissions, but it also suppressed the formation of byproducts, including carbon monoxide.
The exceptional performance of Na-ZSM-5 was underscored by a conversion output four times greater than that achieved via conventional heating methods. This leap in efficiency is attributed to the unique properties of microwave heating, which allowed for localized temperature peaks within the zeolite structure, catalyzing the selective transformation of reactants into desired products without the adverse effects of coking.
A crucial investigation into the molecular dynamics within zeolite during microwave exposure revealed localized temperatures soaring beyond 1000°C, while bulk temperatures remained stable at around 500°C. This phenomenon is pivotal; it highlights how microwave heating could unlock new pathways for enhanced selectivity in olefin production. By effectively manipulating the molecular environment within the catalyst, researchers can catalyze reactions with unprecedented specificity and efficiency.
The implications of this research extend far beyond a simple laboratory achievement. As the need for sustainable industrial practices becomes more pressing, innovations like microwave-assisted catalysis offer a compelling path forward. The ability to derive energy from renewable sources such as solar and wind to power microwave systems could dramatically reduce the environmental footprint associated with chemical production.
With ongoing efforts to fine-tune these microwave-driven catalytic processes, Tsubaki and his team aspire to boost yield and energy efficiency further while exploring larger-scale applications. Their vision suggests a new paradigm in chemical manufacturing—one that embraces sustainability through cutting-edge technology.
In light of the severe challenges posed by climate change and environmental degradation, the research conducted at Kyushu University signifies a pivotal development in the sustainable transformation of the chemical industry. By harnessing the power of microwaves, researchers are not only enhancing the efficiency of biomass conversion but also paving the way for a more eco-friendly approach to producing essential chemicals. As this field evolves, it holds the promise for a revolution in how we synthesize the materials that underpin modern society, ultimately contributing to a more sustainable future.

Leave a Reply