As the world faces an escalating crisis of antibiotic resistance, the importance of developing innovative solutions cannot be overstated. Traditional antibiotics are becoming less effective as bacteria evolve defenses, leading to a critical need for new strategies in our fight against infections. Research from the University of Illinois Chicago has unveiled a remarkable new class of antibiotics known as macrolones, offering a glimmer of hope in this battle. This paper, recently published in *Nature Chemical Biology*, unveils a groundbreaking mechanism that makes it exceedingly difficult for bacteria to develop resistance, a monumental achievement in antibiotic research.
Understanding Macrolones: A New Class of Antibiotics
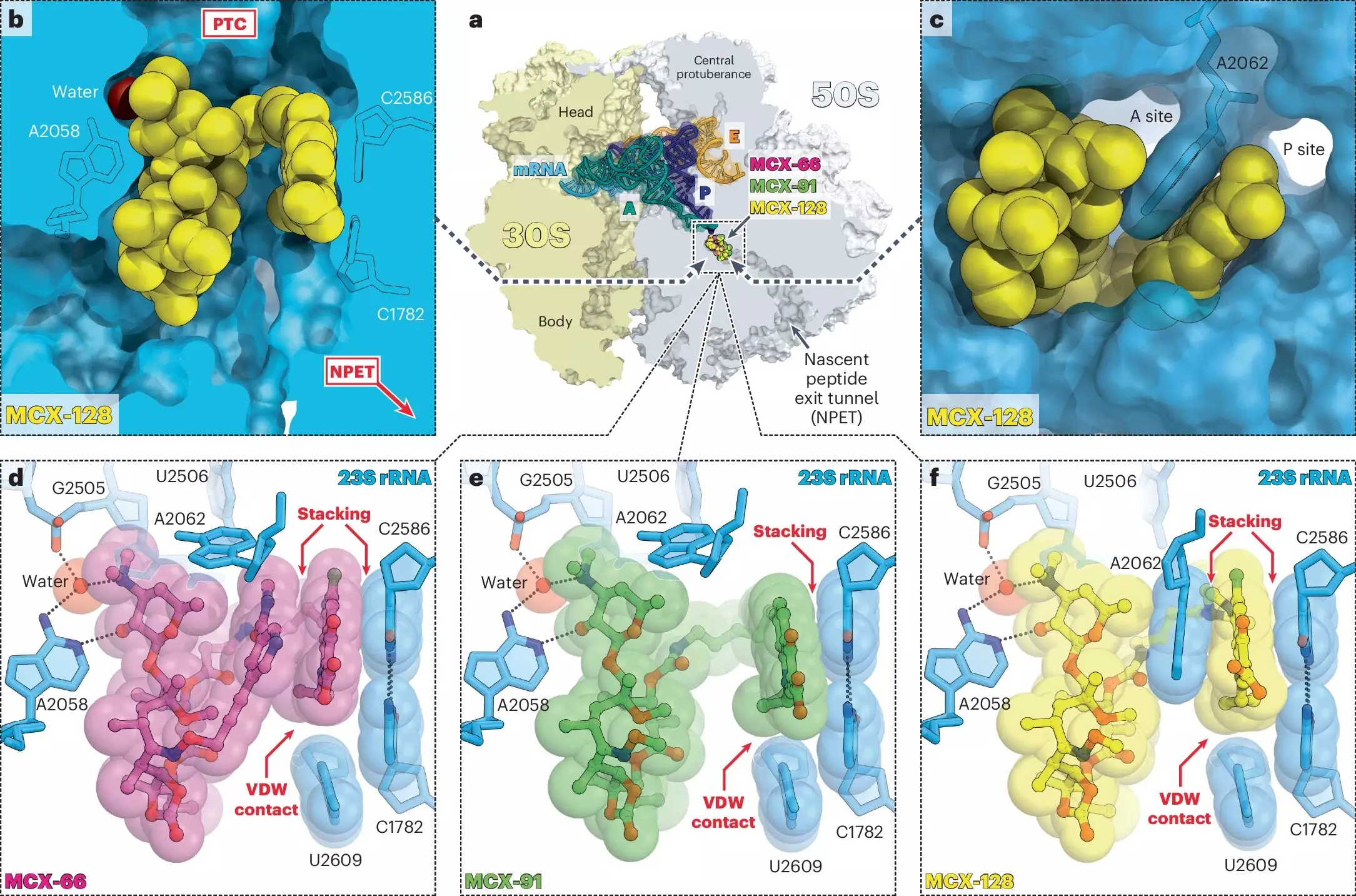
Macrolones are engineered antibiotics that fuse aspects of existing drug classes, specifically macrolides and fluoroquinolones. Traditional macrolides, like erythromycin, are known for blocking bacterial protein synthesis by targeting the ribosome—the cellular structure responsible for manufacturing proteins. On the other hand, fluoroquinolones, such as ciprofloxacin, disrupt DNA replication by targeting the enzyme DNA gyrase. By creating macrolones that attack both of these critical bacterial functions, researchers have taken a revolutionary approach to combating infections.
The ability of macrolones to engage two separate cellular targets is the cornerstone of their potential effectiveness. Researchers, led by esteemed figures like Alexander Mankin, Yury Polikanov, and Nora Vázquez-Laslop, have uncovered that by intercepting bacterial functions at dual points, these drugs sufficiently complicate the evolutionary adaptations that bacteria must undertake to develop resistance. “The beauty of this antibiotic is that it kills through two different targets,” Mankin articulated, underscoring the dual-action approach that differentiates macrolones from previous antibiotic offerings.
Mechanism of Action: How Macrolones Disrupt Cell Functions
The design of macrolones allows them to exploit the inherent weaknesses in bacterial biology. Their experimental applications have revealed that these drugs bind to the ribosome more tightly than conventional macrolides, effectively hijacking the machinery necessary for bacterial protein synthesis. Crucially, this feature extends to strains of bacteria that exhibit resistance to traditional macrolides, showing that macrolones can act where other drugs fail without triggering resistance gene activation.
However, this is not where the innovation ends. Research demonstrated that macrolones exhibit selective inhibition of ribosomes and DNA gyrase at various doses. Remarkably, the most effective macrolone was able to inhibit both targets at the lowest dosages tested. This characteristic is imperative; hitting both targets simultaneously renders the chance for bacterial survival and mutation exceedingly slim. The implications are profound—by reducing the likelihood of resistance evolution, macrolones promise a new frontier in treating infections that currently lay dormant in the shadows of antibiotic ineffectiveness.
The Collaborative Spirit of Discovery
Beyond the scientific brilliance of the macrolones themselves, the interdisciplinary collaboration at the UIC Molecular Biology Research Building is a worthy subject of discussion. This environment fosters innovation by bringing together diverse expertise from various fields—medicine, pharmacy, and biological sciences—creating a dynamic atmosphere ripe for groundbreaking discoveries. Such collaboration proves vital in tackling complex problems like antibiotic resistance, allowing researchers to pool their knowledge and resources effectively.
Mankin remarks on the significance of this collaborative framework, stating, “The main outcome from all of this work is the understanding of how we need to go forward.” This suggests that success in combating bacterial infections lies not just in the tools we forge, but in the unity of the scientific community that drives these discoveries.
Looking Ahead: Optimizing Macrolones for Real-World Application
As the research into macrolones progresses, there remains an imperative to optimize these dual-target antibiotics for clinical use. The potential they hold is a tantalizing prospect, and further study will help refine their application in treatment regimens. The pathways to ensuring that these innovative antibiotics reach the hands of healthcare practitioners worldwide must be carefully navigated, balancing efficacy with the body’s tolerance to these new drugs.
Through continued exploration and a commitment to collaboration, scientists may soon unleash a suite of antibiotics capable of combating resistant strains, restoring faith in our medical arsenal. The advent of macrolones signifies a transformative moment in the fight against bacterial infections, blazing the trail towards a future free from the grip of antibiotic resistance.

Leave a Reply