Recent advancements in quantum biology shed light on Alzheimer’s disease, prompting a profound reevaluation of long-held beliefs about its underlying causes. A novel quantum effect observed in amyloid fibrils—structures known for their association with Alzheimer’s and various neurodegenerative disorders—could herald a paradigm shift in how researchers approach the disease and its potential treatments.
For years, amyloid fibrils have been at the forefront of Alzheimer’s research. These fibrous proteins are the hallmark targets for many experimental treatments, which typically aim to either diminish the number of amyloid deposits or prevent their formation altogether. However, this focus has been met with frustration. Many individuals with substantiated amyloid accumulation never experience dementia, while current therapies targeting amyloid fail to produce the desired clinical outcomes. This inconsistency raises critical questions about the role of amyloid fibrils in the pathology of Alzheimer’s.
The understanding of amyloid fibrils has been overshadowed by the concept of allostatic load, which signifies the cumulative toll of chronic stress and oxidative damage on bodily systems. Elevated oxidative stress has been known to correlate with a higher risk of developing dementia. Researchers began to explore the underlying mechanisms at play, leading to a groundbreaking discovery on quantum effects within biological systems.
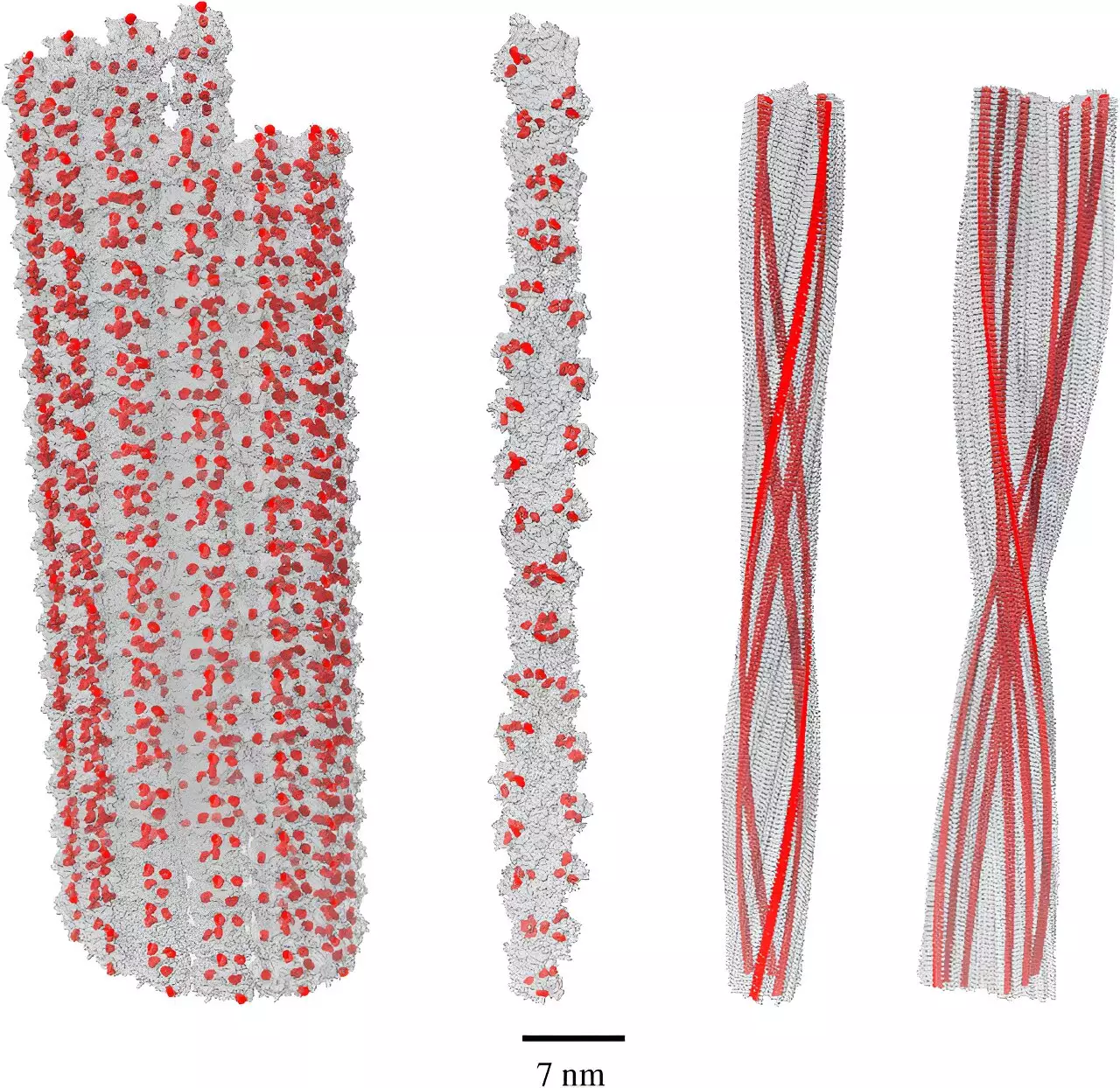
Led by Dr. Philip Kurian at Howard University, recent studies have uncovered a remarkable quantum phenomenon known as single-photon superradiance. This effect, which occurs when molecules can collectively absorb and emit energy efficiently, was previously identified in networks of the amino acid tryptophan. Significantly, Kurian and his research team determined that the collective behavior of tryptophan in amyloid fibrils enhances superradiant effects dramatically, demonstrating a quantum yield five times stronger than that seen in isolated molecules.
This finding holds sophisticated implications for the perception of amyloid fibrils. Rather than perceiving them solely as harmful aggregates contributing to Alzheimer’s disease, it’s now posited that they may serve as a protective mechanism against oxidative stress. The potential for amyloids to absorb high-energy, damaging UV photons produced by free radicals establishes them as a defensive structure within the brain.
The Role of Oxidative Stress and Amyloid as Protection
Oxidative stress plays a central role in neurodegenerative diseases, including Alzheimer’s, suggesting that the body is constantly trying to combat the damaging effects of free radicals. The recent revelations regarding amyloid fibrils suggest they may represent a biological adaptation rather than a primary pathology. By absorbing harmful photons and converting them into lower-energy, safer emissions, amyloid fibrils could act as a protective response to a stressful environment.
This counterintuitive perspective shifts the focus from merely targeting amyloid for reduction or elimination to understanding how these structures contribute to maintaining cellular homeostasis under duress. It challenges the foundational belief that amyloid presence is inherently detrimental, instead proposing that it may reflect an evolved strategy to minimize oxidative damage and preserve neuronal integrity.
The study’s implications resonate through the Alzheimer’s research community. As Professor Lon Schneider noted, Kurian’s findings may overturn the widely accepted assumption that amyloids are the primary culprits in neurodegeneration. If amyloid aggregation is indeed a protective response rather than a causative factor, it necessitates a reengagement with treatment paradigms that consider enhancing this protective role instead of outright dismantling amyloid structures.
Looking ahead, Dr. Kurian emphasizes the importance of bridging theoretical constructs across disciplines including quantum physics, biology, and neuroscience. He envisions a future where the interactions of light and biological systems are recognized for their significance across the landscape of life sciences. Such interdisciplinary collaboration may yield innovative therapies that leverage the body’s innate defense mechanisms rather than undermining them.
The success of this research illustrates the necessity of adopting a broader perspective concerning scientific inquiry. As Mr. Hamza Patwa, a key contributor to the research, points out, truly groundbreaking research often emerges from the intersection of disparate fields, underscoring the importance of a holistic understanding of complex systems.
This breakthrough serves not only as a crucial piece in the puzzle of Alzheimer’s etiology but also as a reminder that scientific progress thrives on innovative thinking and the willingness to challenge existing dogmas. As new research unfolds, the integration of quantum biology into neuroscience may illuminate further mysteries of the human brain and guide future therapeutic approaches that prioritize protection over destruction. The journey to understand Alzheimer’s may be reshaped by these quantum insights, potentially leading to novel interventions that could fundamentally change the landscape of neurodegenerative disease management.

Leave a Reply