The immunoproteasome plays a critical role in the immune system’s response to pathogens such as viruses and bacteria. This enzymatic complex is instrumental in processing and presenting antigens, allowing immune cells to recognize and combat infectious agents effectively. However, the overactivity of immunoproteasomes in certain conditions, particularly autoimmune diseases, leads to a dire miscalculation where the body’s immune system turns against its own healthy cells. This pathological process has significant implications, necessitating the development of therapies that specifically target the immunoproteasome without affecting other proteasome functions essential for cellular homeostasis and protein degradation.
For years, scientific endeavors have sought to discover selective inhibitors that can modulate the activity of the immunoproteasome. A significant challenge in this quest is the need to distinguish between the diverse proteasome variants that perform a range of critical cellular functions, including protein recycling and waste management. Any inhibitor that is not selective can inadvertently disrupt these processes, leading to unintended side effects that may exacerbate rather than alleviate the condition being treated. Thus, achieving specificity in immunoproteasome inhibition is not merely desirable—it is essential for the development of safe and effective therapies for autoimmune diseases.
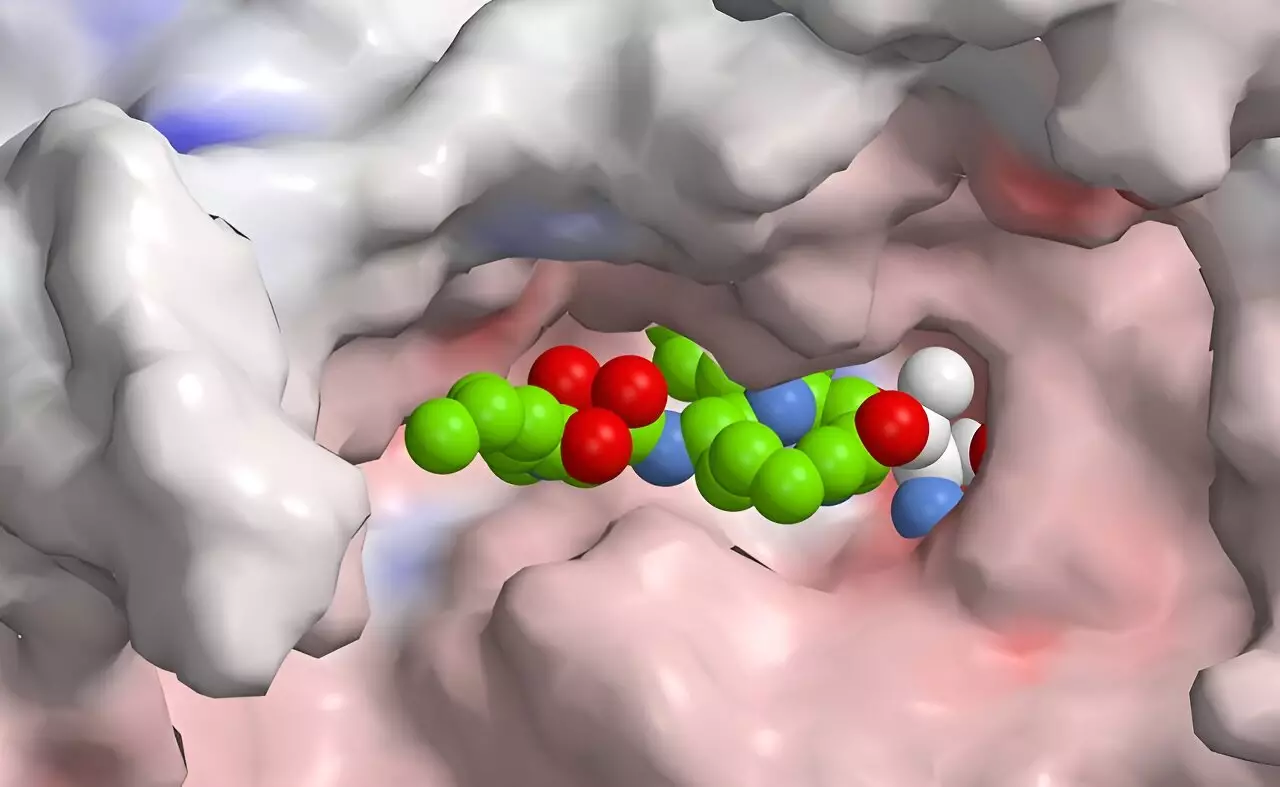
Led by Helge Bode at the Max Planck Institute for Terrestrial Microbiology in Marburg, a collaborative research team has embarked on a promising journey to address this challenge. Utilizing synthetic biology and innovative engineering techniques, Bode and his colleagues have adeptly manipulated the production of natural bacterial compounds, resulting in the development of a new drug candidate that demonstrates higher selectivity for the immunoproteasome. Their research illuminates the fascinating intersection of peptides and polyketides in drug development, broadening the scope of naturally derived compounds available for therapeutic applications.
What sets their research apart is the introduction of a peptide-polyketide hybrid—a structural combination that could revolutionize how immunoproteasome inhibitors are designed. By leveraging a novel approach known as XUT technology, the team successfully fused two types of enzyme complexes, paving the way for creating tailored therapeutic agents that exploit the advantageous qualities of both peptides and polyketides.
Harnessing Nature’s Designs
Interestingly, the natural world offers insights that can inform synthetic strategies. The research team drew inspiration from naturally occurring hybrids produced by microbes, particularly a group known as syrbactins. These compounds, which effectively inhibit proteasome function in various organisms, have garnered attention for their potential in cancer therapy, as their mechanism induces apoptosis in tumor cells by impairing cellular waste disposal processes. However, no specific immunoproteasome inhibitors derived from syrbactins had been developed—until now.
Through meticulous rational design, Bode’s team is working to modify syrbactin structures to yield optimally selective inhibitors that target the human immunoproteasome. While their current compound demonstrates promising characteristics, researchers acknowledge that further refinement is necessary to enhance selectivity and minimize unwanted systemic effects.
The Future of Drug Development
As the search for selective immunoproteasome inhibitors continues, the future of drug development appears increasingly bright. With advancements in computational methods and high-throughput screening processes, researchers are poised to identify and optimize a diverse array of compounds that can be tailored for specific disease applications. The goal is not only to create effective therapeutic agents but also to ensure they can be administered with minimal risk to the patient’s health.
The work spearheaded by Helge Bode and his team is a significant leap forward in the fight against autoimmune diseases. By embracing the complexities of enzyme interactions and tapping into the rich reservoir of natural compounds, there lies a path to innovative therapies that promise better outcomes for countless individuals struggling with these debilitating disorders. As research progresses, we can anticipate a new era in targeted immunotherapy, where precision and efficacy converge to restore health and balance in the immune system.

Leave a Reply