Fuel cells stand at the forefront of sustainable energy solutions. By converting hydrogen and oxygen into electricity through electrochemical processes without combustion, they offer a significant advantage in reducing air pollution compared to conventional energy sources. From powering electric vehicles to providing energy for industrial applications, the potential uses of fuel cell technology are vast. However, the high costs associated with the materials and catalysts currently used in many fuel cell designs present a barrier to more widespread implementation. Recent advancements in anion-exchange-membrane fuel cells (AEMFCs) provide a glimmer of hope in overcoming these challenges by relying on non-precious, Earth-abundant materials.
Traditional fuel cell designs use expensive catalysts, often based on precious metals like platinum, raising significant concerns regarding cost and sustainability. These precious metal components not only drive up manufacturing expenses but also pose supply chain vulnerabilities. Given the rising demand for more sustainable energy technologies, researchers have recognized the need to explore alternatives that employ cheaper materials without sacrificing efficiency and effectiveness.
One promising avenue arises from advancements in AEMFCs, which utilize low-cost catalysts while maintaining the potential for high energy output. However, these favorable attributes are often negated by the self-oxidation phenomenon that affects non-precious metal catalysts, leading to significant performance degradation over time. Developing solutions that prevent this form of degradation has become a pivotal focus in research efforts across the globe.
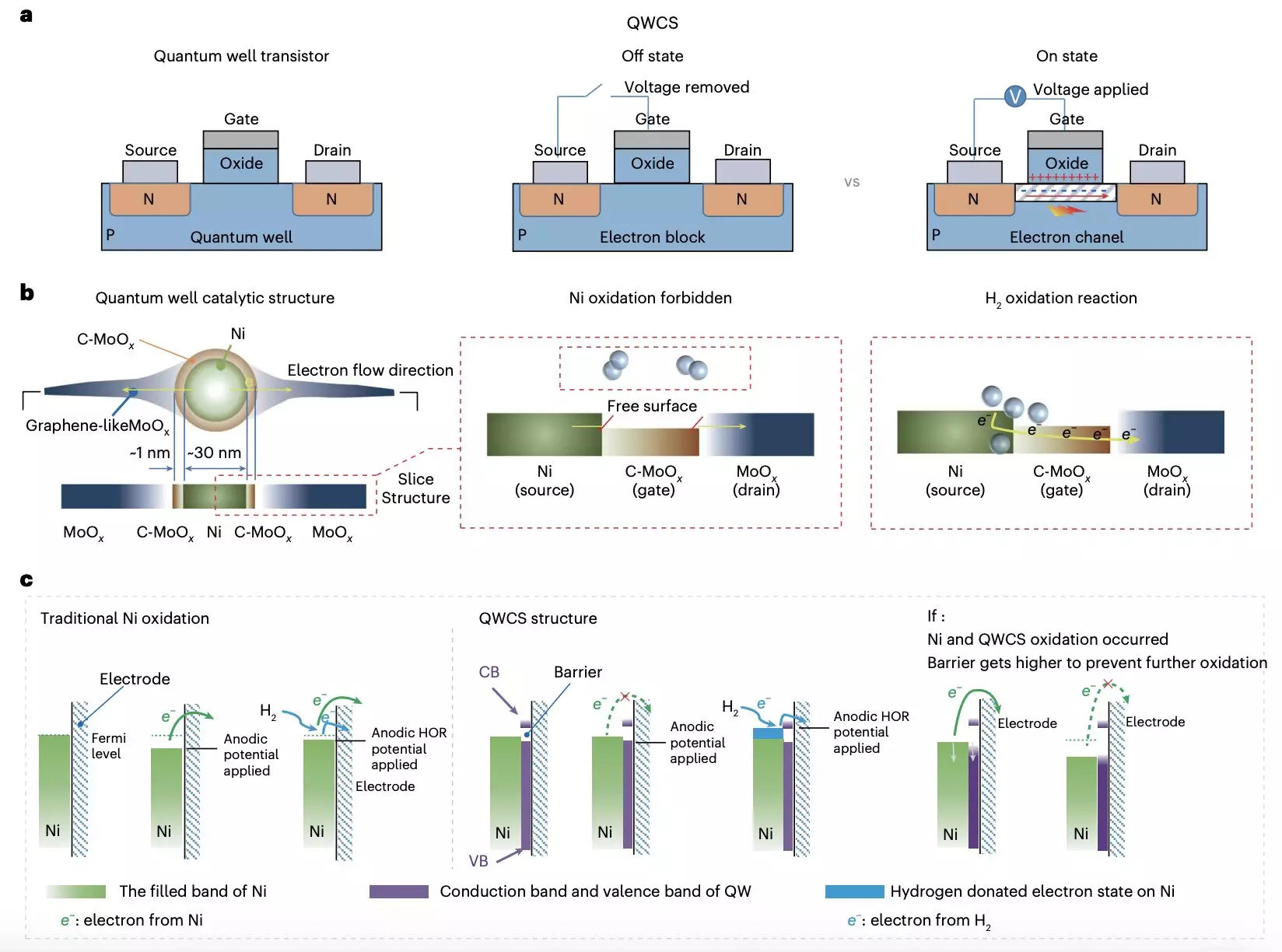
Researchers from Chongqing University and Loughborough University have introduced a groundbreaking strategy that seeks to enhance the durability and performance of metallic nickel catalysts in AEMFCs. By employing a unique quantum well-like catalytic structure (QWCS), they have designed nanoparticles of metal nickel that can effectively resist self-oxidation—a common flaw of non-precious metal catalysts. As detailed in their recent publication in Nature Energy, this innovative approach comprises a heterojunction framework that represents a significant leap in fuel cell technology.
The QWCS is engineered to enhance catalytic activity and maintain high stability in adverse conditions. Specifically, it consists of atomically confined nickel nanoparticles positioned within a carbon-doped-MoOx/MoOx heterojunction. This configuration creates a barrier that allows electrons generated during the hydrogen oxidation reaction to be selectively transferred without allowing the nickel catalyst to degrade from its metallic state, effectively enhancing overall cell longevity.
The newly developed Ni@C-MoOx catalyst demonstrates remarkable stability, maintaining performance after exhaustive testing, including over 100 hours of continuous operation. The innovative structure is not only successful in sustaining catalytic activity but also exhibits a high specific power density of 486 mW mgNi⁻¹, a significant achievement that underlines its practicality for real-world applications. Furthermore, it boasts resilience even under conditions of hydrogen starvation, demonstrating its reliability for commercial use where fuel supply does not remain constant.
The team’s findings indicate that the electronic configuration provided by the QWCS inherently protects the Ni catalyst through a barrier of 1.11 eV. This technological advancement shines a light on the potential to utilize quantum confinement in developing catalysts that could redefine the standard for AEMFCs.
The implications of this discovery extend beyond merely enhancing AEMFC performance. The underlying design principles and innovative applications of quantum technologies present a fertile ground for the advancement of alternate catalysts, moving toward a future where sustainable energy solutions can become more economically viable. The work of the research groups at Chongqing University and Loughborough University could potentially lead to more reliable, efficient fuel cell systems with improved durability and lower costs, paving the way for broader application across various sectors.
If successful, this strategy may create a ripple effect, encouraging further research and investment in non-precious metal catalysts. By continuing on the path of innovation and collaboration, the potential for fuel cells to significantly contribute to energy sustainability and environmental protection could become a reality, heralding a new era of clean, efficient energy.

Leave a Reply