From the groundbreaking inventions of Thomas Edison to contemporary technological advancements, the journey of innovation has been profoundly influenced by trial-and-error experimentation. Edison’s rigorous search for the ideal filament to create a viable lightbulb serves as a historical benchmark for the scientific process, demonstrating how iterative research often fuels progress. Fast forward to the present day, and this same method remains prevalent, significantly impacting the development of battery systems, which underpin modern convenience and sustainability. However, while experimentation is crucial, contemporary scientific endeavors necessitate a deeper understanding of the principles governing material functionality. This dual approach paves the way for the creation of enhanced materials capable of meeting increasingly challenging demands.
An important stride in this complex area of study has been highlighted in a recent paper published in the *Proceedings of the National Academy of Sciences*. Researchers from the University of Delaware, Northwestern University, and various industry specialists have uncovered valuable insights into the behavior of electrons within complex fluids—specifically, slurries utilized in electrochemical devices like batteries. This collaborative research effort, led by notable figures such as UD’s Norman Wagner and Northwestern’s Jeffrey Richards, has tackled existing gaps in knowledge about electron transport in these materials.
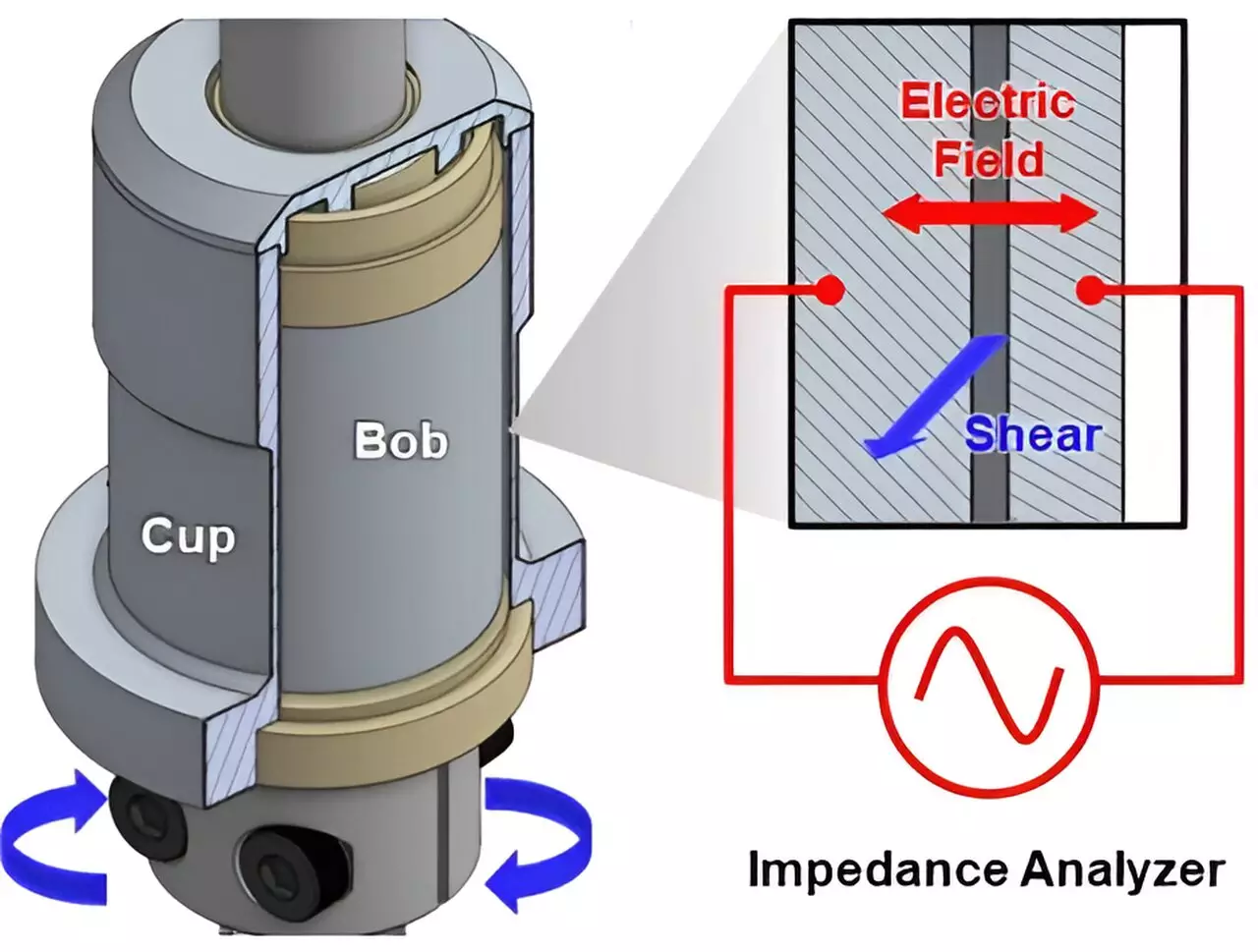
Wagner emphasizes that improving the performance of electrochemical devices necessitates much more than simply refining chemical formulations; it requires an intricate understanding of how electrical conductivity is affected during the manufacturing and processing of slurry materials. The research underscores the indispensable relationship between microstructure—how various components within a material assemble—and the flow of electrons, a factor that significantly determines device efficiency and power output.
To provide further clarity, let’s consider the example of batteries, which convert chemical energy into electrical energy through electron movement within a slurry composed of conductive materials. Similar to racecars on a track, many devices share fundamental components—such as conductive pathways and reactive elements—yet differ in subtle ways that affect overall performance. The microstructural details in the composition of a slurry can create disparities in how effectively electrons are able to navigate through the conductive particles.
In contemporary battery technology, carbon black—entailing nano-sized aggregates of carbon crystals—has emerged as a crucial ingredient, facilitating the conductivity required for efficient performance. However, as Wagner elaborates, the slurries must be scrutinized as dynamic systems; even though electrons can transfer quickly within carbon black, they must hop between distinct clusters suspended in a liquid. This hopping mechanism introduces a layer of complexity that researchers seek to decode thoroughly.
In this latest study, the research team has developed a comprehensive framework aimed at elucidating how the conduction properties of flowing slurries are contingent upon their component chemistry and processing. This pioneering roadmap stands to revolutionize the manufacturing processes for energy storage devices. The findings elucidate how the kinetic behavior of slurries can influence device performance, inviting fresh insights into how to optimize material inputs at the manufacturing stage.
Wagner states, “What we’ve studied allows us to begin to understand how the structure of this carbon-black slurry impacts the efficiency and performance of these devices.” While the research does not directly resolve specific issues facing individual battery technologies, its foundational insights offer the potential for broader applications across various electrochemical systems, allowing for tailored solutions in energy storage.
The significance of this research transcends just battery technology; its implications can also extend to emerging technologies such as electrolyzers, which separate water into hydrogen and oxygen. This complex electrochemical process hinges on the ability to manipulate the solution’s material properties effectively—a challenge that aligns closely with the insights provided by Wagner and his team.
As Wagner notes, “You can get the chemistry right, but if you don’t process it right, you don’t end up with the performance that you want.” This highlights the integral balance between achieving optimal chemical formulations and ensuring the physical processing of materials sets the stage for the desired technological outcomes.
Through groundbreaking studies like these, the prospective future of material science and electrochemical device efficiency appears promising. By refining our understanding of how conductive materials function—how they are formulated, processed, and ultimately utilized—the barriers to achieving higher performance levels in energy storage technologies can gradually be dismantled. As researchers continue to explore these foundational principles, the path toward innovative solutions that meet modern energy demands will become clearer and more attainable.

Leave a Reply