Nitrate contamination in water sources has emerged as a critical issue that poses severe risks to both human health and the environment. This issue is particularly alarming as high nitrate levels in drinking water are linked to various health problems, including methemoglobinemia, often referred to as “blue baby syndrome.” Furthermore, the environmental consequences are stark, with excess nitrates contributing to eutrophication in aquatic systems, leading to harmful algal blooms. Addressing this growing concern has become imperative, and recent research from Yale University may hold the key to significantly advancing our capabilities in nitrate removal.
Innovative Solutions: Electrified Membranes
Lea Winter, an assistant professor of chemical and environmental engineering at Yale, has spearheaded a groundbreaking approach involving electrified membranes constructed with carbon nanotubes. Traditional methods of removing nitrates from water typically revolve around either separating or destroying the pollutants. Winter highlights the inefficiencies of merely separating nitrates, as this approach culminates in a waste byproduct that can ultimately re-enter our water systems if not handled properly.
Instead of relying solely on separation, the focus on destruction presents a more sustainable avenue. Traditional biological denitrification methods, while effective in specialized settings like wastewater treatment plants, are hampered by their sensitivity to environmental shifts such as temperature and chemical composition. This sensitivity, coupled with a reliance on microbial activity, results in a sluggish process that desperately needs innovation.
Electrocatalysis: Speeding Up the Process
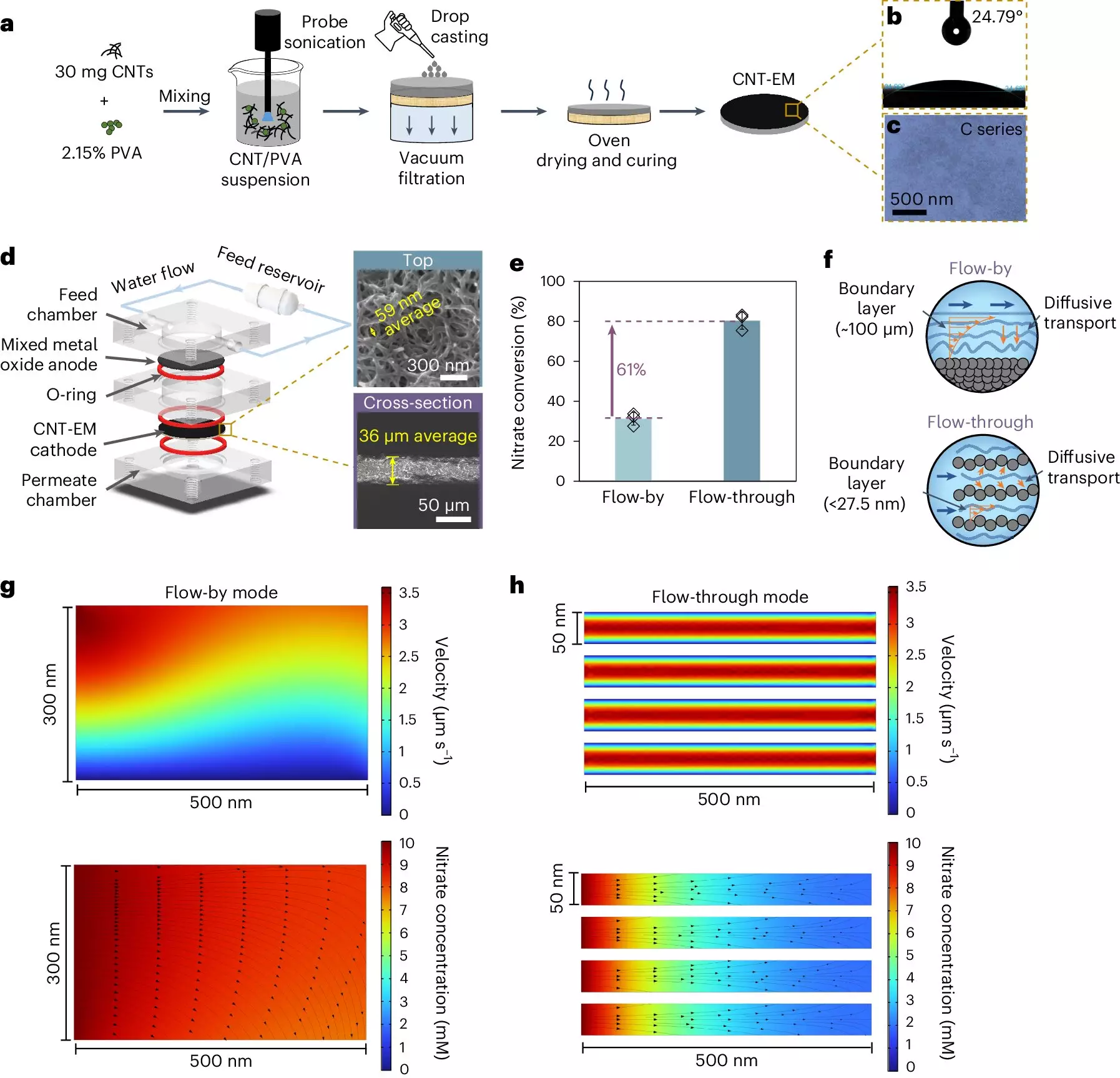
To remedy the slow kinetics associated with biological approaches, researchers have turned to electrocatalytic processes, which operate more swiftly and effectively degrade nitrates into benign substances. Initially, conventional electrochemical systems, which employ flat plate electrodes, struggled to efficiently react nitrates due to the cumbersome fluid dynamics involved. The “boundary layer” that forms around electrodes presents a challenge: a thicker layer means slower transport rates for nitrates, thus diminishing the reaction efficiency.
Winter’s laboratory has risen to this challenge with an innovative solution: electrified membranes comprised of carbon nanotubes embedded in a polymer matrix. These membranes feature exceptionally small pore sizes—about 50 nanometers, which are 2000 times smaller than those found in traditional systems. This revolutionary design not only promotes faster transport of nitrates to the electrode surface but also optimizes the overall reaction rate.
Efficiency Without Metals: A Breakthrough Discovery
What sets this new approach apart from conventional electrochemical systems is its elimination of metal components, which are typically crucial for enhancing nitrate conversion. By leveraging the unique properties of carbon nanotubes as catalysts, Winter’s membranes achieve nitrate conversion rates comparable to their metal-containing counterparts. The implications of this development are striking: where traditional techniques often require hours for significant removal rates—from 80% to 90%—Winter’s process accomplishes similar outcomes in a mere 15 seconds.
This dramatic reduction in processing time could revolutionize how we approach water treatment and set a new standard for efficiency in the field. The benefits extend beyond simply faster results; by minimizing the time wastewater interacts with nitrate contaminants, it allows for a sleeker integration of water treatment processes that can be adapted to existing infrastructure.
From Lab to Real-World Application
To test the viability of this innovative technology, Winter and her team sampled water from Lake Wintergreen, near Yale’s campus, introducing controlled nitrate concentrations into the mix. The ability to treat lower concentrations—which are more representative of those found in contaminated water sources—is vital for real-world sustainability.
This experiment is not just an academic exercise; it signals a potential shift in how we tackle nitrate pollution at larger scales. The practical applications of Winter’s technology could empower municipalities and industries that have faced chronic nitrate contamination challenges.
By paving the way for rapid and efficient nitrate removal, this research will contribute significantly to ensuring safe drinking water and protecting our delicate ecosystems. The implications of this breakthrough could have lasting effects on public health and environmental stewardship.
As we move forward into an era marked by growing environmental challenges, innovative solutions like Winter’s electrified membranes represent a beacon of hope that could redefine water safety standards.

Leave a Reply